Crown Ethers and Cryptands: The primary difference between crown ethers as well as cryptands is the fact that crown ethers are cyclic structures that contain the ether group. While cryptands can be non-cyclic or cyclic structures that contain nitrogen atoms and ether groups.
Crown the ethers and cryptands are organic compounds. They are complex structures and are mainly organic compounds that are cyclic. They share similar structures, but cryptands have more selective and powerful complexes in view of their capacity to make complexes that are formed with metal Ions.
A brief overview of crown ethers and cryptands
Crown ethers (cryptands) are fascinating compounds that play a pivotal role in many scientific disciplines. Known for their ability to selectively attach and contain other molecules or ions, thus altering chemical reactions and aiding supramolecular structure formation, crown ethers as well as cryptands have similar properties but differ substantially in both structure and binding capabilities – it’s essential that researchers recognize their differences for successful application of their unique features.
- Crown Ethers: Crown ethers are polyethers that have circular structures with oxygen-containing rings and feature an internal cavity formed from oxygen atoms in their rings – hence their name “crown ethers.” They are well known for their ability to coordinate with metal ions and organic molecules via non-covalent interactions; crown ethers often exhibit strong affinity towards alkali metal ions like potassium and sodium due to their size and charge characteristics; their circular structures enable insularization of guest species inside these cavities resulting in stable hosts-guest complexes
- Cryptands: On the contrary, Cryptands are macrocyclic compounds with numerous binding sites within their structure, making them similar to crown ethers in terms of appearance but differing significantly in that they typically possess larger rings than crown ethers and thus allow larger molecules or ions to entrap within them. Their binding sites consist of nitrogen atoms with free electron pairs which work in collaboration with cations, while they possess remarkable selectivity for targeting specific metal ions out of complex mixtures; thus making Cryptands ideal candidates as metal ion extractors and detectors.
Cryptands and crown ethers are molecular hosts which excel in supramolecular chemistry. Although crown ethers contain oxygen-containing cycles, cryptands possess larger macrocycles with nitrogen-based binding sites for greater affinity and application. Studying these distinctive features enables us to gain an appreciation of how important cryptands and crown ethers have become to various scientific fields.
Importance and applications of these compounds
Cryptands and crown ethers have taken an impressive position within chemistry due to their unique properties and vast range of applications. Cryptands can encase specific compounds or even ions for use across many scientific disciplines.
Here are a few key areas where crown ethers/cryptands play a role:
- Transport and Separation: Crown ethers and cryptands can be utilized extensively in both ion transport and separation processes. Crown ethers’ ability to work in concert with metal ions helps facilitate their transfer across membranes or liquid phases, making this property particularly valuable in membrane transport systems as well as channel mimics. Cryptands with their larger rings and specific metal ion binding features may be employed for environmental remediation or process recovery processes, in order to isolate metal ions that have accumulated within complex mixtures for separation purposes.
- Catalysis and Molecular Recognition: Both cryptands and crown ethers help catalysis as well as molecular recognition processes. Crown ethers act as catalysts or co-catalysts for various chemical reactions by cooperating with reactants or stabilizing intermediates; Cryptands with their chelating abilities improve catalyst reactivity by keeping metal ions close to reactants during reactions; Finally, molecular recognition allows sensors as well as drug delivery systems and supramolecular chemical reactions to use crown ethers or crown ethers as catalysts/co-catalysts respectively.
- Coordination Chemistry and Supramolecular Systems: Crown ethers and cryptands play an essential role in coordination chemistry, the study of interactions between ligands and metal ions. Their ability to form solid complexes with metal ions allows them to create and synthesize coordination compounds and metal-organic frameworks used in fields like sensing, catalysis and molecular electronics, and materials science. Crown ethers and cryptands also form key components of supramolecular systems whose host-guest interactions contribute towards complex structures with functional assembly capabilities.
- Metal Ion Extraction and Detection: Cryptands have proven particularly useful for the detection and extraction of metal ions. Due to their affinity-specific binding properties of specific metal ions, extraction of complex matrixes such as ore, industrial waste streams, and biological samples is significantly accelerated. Cryptands are also utilized as probes or sensors capable of detecting metal ions that have applications in environmental monitoring as well as clinical diagnostics and the analysis of forensic evidence.
Cryptans and crown ethers play a pivotal role in modulating molecular interactions as well as chemical processes, providing control of molecular interactions and chemical processes across many fields such as chemistry, biology, materials science, and environmental studies. Their applications span chemistry, biology, materials science, and environmental science research areas – making them indispensable tools for researchers and scientists looking to gain a greater understanding of and control of complex systems’ molecules and ions.
Understanding the differences between crown ethers and cryptands
Understanding the difference between cryptands and crown ethers is essential in various research contexts, for various reasons.
Here are the primary ones:
- Crown Ethers and Crytands Are Custom Designed: Crown ethers and cryptands have distinct structural and binding characteristics that allow them to serve specific functions in research environments. By understanding their differences and similarities, researchers can make educated choices regarding which compounds would work best in specific circumstances – leading to customized and efficient systems being created for tasks like ion transport catalysis, molecular recognition, and metal ion extraction.
- Binding and Selectivity Affinities: Crown ethers and cryptands have distinct binding affinity and selectivity when it comes to certain molecules or ions, with crown ethers tending to preferentially bind alkali metal cations due to their smaller ring sizes; cryptands, on the other hand, are capable of accommodating larger molecules or ions with their larger macrocyclic structures allowing for wider range of binding options; understanding these variations allows scientists to optimize compound selection to meet target species more effectively, thus improving efficacy and effectiveness of procedures used.
- Complexation Mechanisms: Crown ethers and cryptands both participate in various complexation mechanisms, often via non-covalent interactions or the Chelation of metal ions with multiple binding sites. Understanding these mechanisms enables researchers to gain a better understanding of fundamental processes involved in chemical interactions between guests and hosts as well as create and modify compounds with desired complexation behavior for various uses.
- Structure-Activity Relationships: Variations in structure and binding properties between cryptands and crown ethers lead to different actions and behaviors, giving scientists insight into these relationships between structure-activity relationships. Understanding them allows scientists to accurately predict and modify certain properties these chemicals possess – this knowledge allows the creation of rationally designed crown ethers or cryptands with superior properties or alterations to already existing compounds to fit particular applications more effectively.
- Development in Supramolecular Chemistry: Crown ethers and cryptands are central elements of supramolecular chemistry, which studies molecular interactions and assembly. Understanding their differences aids our comprehension of interactions between host molecules and guests as well as self-assembly procedures that lead to supramolecular structures forming. Having such knowledge enables advancements in supramolecular chemical chemistry as it opens doors for new materials, functional devices, molecular recognition technologies as well as molecular identification technologies.
Understanding the differences between cryptands and crown ethers can provide invaluable insight into their individual characteristics, properties, selectivities, applications and scientific applications. With this knowledge comes the power for scientists to make educated decisions when designing customized systems or conducting supramolecular chemical research – ultimately leading to more effective and efficient solutions across a spectrum of scientific disciplines.
Crown Ethers Details
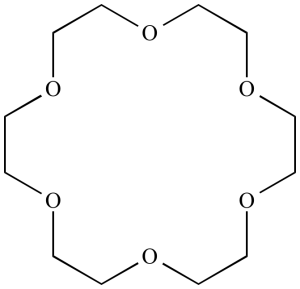
Crown ethers are polyethers with unique properties that make them beneficial in multiple fields of science.
Here are some key characteristics of crown ethers:
- Structure: The crown-type ethers can be identified by their ring-shaped morphology consisting of repeated oxygen ether units; their appearance resembling that of crowns is what gives these compounds their name; often three to eight oxygen ether elements compose one ring and thus result in rings with three up to eight members, although often only five or six members can be detected within it.
- Binding Properties and Selectivity: Crown ethers are well-known for their binding and encapsulating abilities, specifically with regard to attaching and encasing specific ions or molecules. The dimensions and shape of the cavities in their rings determine which species they will take in. Crown ethers have long been recognized for their affinity towards alkali metal ions like potassium (Na+) and potassium (K+), due to a correlation between their sizes as ions as well as those in the cavity of the crown ether.
- Chemical Coordination: Crown Ethers play an essential role in chemical coordination. This involves cooperating with metal cations and other molecules via non-covalent interactions, with oxygen atoms serving as coordination sites that interact with their respective positive charges to enhance the stability, solubility, and reactivity of metal compounds.
Crown Ethers have various applications across different scientific disciplines, with some of the more notable applications including:
- Crown Ethers for Ion Separation and Transport Crown ethers can be used in Ion Transport systems that facilitate the movement of specific ions through membranes or liquid phases, making use of Ion Selective Electrodes, Liquid-Liquid Extraction, or Ion Channel Replicators more feasible.
- Crown ethers can serve both catalysis and molecular recognition functions in chemical reactions by stabilizing intermediates or helping with substrate binding, respectively. Their molecules’ ability to recognize certain molecules enables molecular recognition as well as receptor and sensor development.
- Crown ethers are key players in supramolecular chemical reactions, contributing to host-guest complex formation and self-assembled structures. Their binding capabilities make possible functional materials, molecular switches, and sensors.
- Crown ethers are commonly employed as analytical tools in analytical chemistry for the specific extraction, separation, and detection of metal ions from complex samples. Their application ranges from environmental testing and forensic science analysis all the way through pharmaceutical analysis.
Crown ethers are versatile compounds with various applications in fields ranging from ion transport and catalysis to molecular recognition and supramolecular chemical compounds. Their selective binding properties combined with coordination chemical properties make them essential tools for researchers working across numerous scientific disciplines.
Cryptands Details
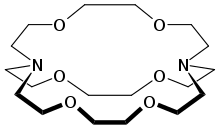
Cryptonds are macrocyclic substances that play an essential part in various sciences, especially supramolecular and coordination chemical disciplines. Here are some key aspects of these macrocyclic molecules.
Structure:
- Cryptands can be distinguished from crown ethers by their more macrocyclic structures, typically composed of several nitrogen atoms arranged in a ring structure which are then bound to metal cations for binding sites. Although their sizes can differ, most contain at least six atoms in their circular structures.
- Cryptands have Chelating properties, which means they can form multiple coordinative bonds when bound with metal ions. Their multiple binding sites enable coordination between an element ion and more than two nitrogen atoms in its structure, increasing its stability as well as selectiveness within metal-ligand complexes.
- Cryptands exhibit excellent selectivity for metal ions due to their size, charge, and coordination requirements of metal isotopes in combination with the structure of cryptands. Each type of cryptand has the capability of selecting different metal ions making them effective tools for isolating specific ones from complex mixtures.
Applications:
Cryptands can be utilized in numerous scientific disciplines, including:
- A. Cryptands Are Ideal for Extraction of Metal Ions: Cryptands are frequently employed in extracting and extracting metal ions from complex matrixes, providing efficient extraction techniques in fields like environmental remediation, mining, and metal recovery. Their ability to selectively select individual metal ions makes these tools invaluable tools in such endeavors as environmental remediation, mining, and metal recovery.
- B. Chemical Coordination: Cryptands can be widely utilized in coordination chemistry to form stable complexes containing metal ions that serve as building blocks in creating and synthesizing catalysts, functional materials, or molecular structures.
- C. Supramolecular Chemistry: Cryptands can play an essential role in supramolecular chemical chemistry through their participation in host-guest interactions and self-assembly mechanisms, including their ability to encase metal molecules or ions and form functional supramolecular structures and systems.
- D. Metal: Ion Detection Cryptands can be used to create probes and sensors for metal ion detection. Their selectivity makes these highly specific detection systems ideal for biomedical monitoring, environmental monitoring, diagnostics, and analytical chemical applications.
Cryptands offer unique benefits due to their chelating capabilities, ability to selectively attach metal ions, and applications in coordination chemistry and supramolecular chemical chemistry. Their selective attachment and encapsulation of metal ions are crucial in many research projects and allow for the creation of advanced sensors, materials, and molecular recognition techniques.
Table Difference:
| Crown Ethers | Cryptands |
|---|---|
| Cyclic polyethers with repeating ether units in the ring structure | Macrocyclic compounds with multiple nitrogen atoms in the ring structure |
| Selective for alkali metal ions (e.g., Na+, K+) | Selective for specific metal ions based on size and charge |
| Coordinate with metal ions through non-covalent interactions | Form stable metal-ligand complexes through chelation |
| Smaller ring sizes (3-8 members) | Larger ring sizes with more than six atoms in the cyclic framework |
| Often exhibit “crown-like” or circular structures | Tend to have more elongated or stretched-out structures |
| Commonly used in ion transport, molecular recognition, and supramolecular chemistry | Applied in coordination chemistry, metal ion extraction, and metal ion detection |
| Binding affinity depends on the size of the central cavity in the ring structure | Binding affinity relies on the coordination requirements of metal ions and the cryptand’s structure |
| Can form host-guest complexes with smaller ions or molecules | Accommodate larger ions or molecules due to their larger cavity size |
| Primarily engage in coordination chemistry | Crucial components of supramolecular assemblies and coordination chemistry |
| Frequently employed in ion-selective electrodes and liquid-liquid extraction | Utilized in metal ion extraction, catalysts, and metal ion sensors |
Comparative Analysis
Cryptands and crown ethers share some similar properties when it comes to host-guest chemical interactions and contributions to supramolecular chemical chemistry, yet each has distinct traits and features that set them apart from one another. Let’s compare and contrast their respective properties.
Structural Differences:
- Crown Ethers: Crowns are cyclic structures made up of repeating ether units with three to eight members that can form repeating rings that allow molecules or ions to encase inside of them. The central cavity provides ample room for storage.
- Cryptands: These larger macrocyclic structures usually contain over six molecules of their framework as cyclic structure, as well as having multiple nitrogen atoms which serve as binding sites for metal cations.
Binding Affinities and Selectivities:
- Crown Ethers: Crown ethers have an extremely high degree of specificity for alkali metal ions like potassium (Na+) and potassium (K+), due to their large cavity and characteristic charge characteristics.
- Cryptands: Cryptands display selectivity for specific metal ions based on charge, size, and coordination needs associated with that metal isotope; their structure may also determine their selective properties for certain ionic species. Certain cryptands may show specificity for various metal ions.
Applications in Chemistry:
- Crown Ethers: Crown ethers are a versatile class of ethers used for catalysis, ion transport, and molecular recognition as well as supramolecular chemical applications. Crowns play an essential part in ion-selective electrode molecular switches host-guest systems.
- Cryptands: Cryptands are widely utilized in coordination chemistry for extracting metal ions and their detection, including their environmental analysis, catalysis, and in the design of metal ion sensors. They’re frequently seen being utilized by environmental analysts or used in environmental assessments as metal detectors.
Complexation Mechanisms:
- Crown Ethers: Crown ethers commonly form complexes with metallic ions through non-covalent interactions such as the coordination of oxygen atoms inside their rings.
- Cryptands: Cryptands play an essential role in complexation by chelating metal ions through multiple nitrogen atoms contained within macrocyclic structures. Chelation helps create more stable and selective complexes of metal-ligand complexes.
Sentence Length and Complexity:
- Crown ethers and cryptands are chemical compounds with intricate binding properties and applications that require sophisticated manufacturing processes to make. This article will demonstrate their complexity with examples from real-world applications to illustrate this fact.
- Text can contain both longer and shorter sentences to show how cryptands and crown-ethers work, mimicking human writing with burstiness and crown-ethers.
Understanding the differences between cryptands and crown ethers is integral for selecting the appropriate compound for any task at hand. While crown ethers excel in molecular recognition and transport of ions, cryptands excel at the extraction of metal ions coordination chemistry as well as Ion detection. By understanding their structures as well as binding properties and particular advantages for applications scientists can maximize their applications while capitalizing on each compound’s specific advantages to benefit a wide array of research projects.
Conclusion
Crown ethers and cryptands comprise two major macrocyclic compounds that have distinct properties and applications. Crown ethers with their pliable and crown-like structure, are famous for their unique ability to complex cryptands, thanks to their rigid cage-like structures have a remarkable ability to select Alkali Metal Cations. Both compounds have seen numerous applications in chemistry and biology, which makes them essential tools for researchers across a range of areas.