Amino acids are the basic building blocks of life. which play an important role in the biological processes that sustain us. These remarkable molecules are chiral, meaning they exist in two mirror-image forms. left-handed (L-amino acids) and right-handed (D-amino acids). Understanding the difference between Left and Right Handed Amino Acids acid types is essential to understanding their different functions in living organisms. In this comprehensive guide, we’ll explore the intriguing world of amino acids. Let’s focus on the difference between left and right-handed variants.
Importance of Left-Handed Amino Acids
Left-handed amino acids, also known as L-amino acids. Most important in biology and biochemistry due to their exclusive role in building the primary structure of proteins. Proteins are the workhorses of biological systems.
which perform many essential functions including enzymatic catalysis, structural support, and molecular signaling. The unique chirality of L-amino acids ensures the precise folding and three-dimensional structure of the protein. enable them to perform their designated tasks effectively.
Any inclusion of their right-handed counterparts, D-amino acids, will lead to distorted and non-functional protein structures. So, the predominance of L-amino acids in protein synthesis is critical to life as we know it, highlighting their fundamental role in the complex machinery of organisms.
Importance of Right-Handed Amino Acids
Right-handed amino acids, or D-amino acids. plays a relatively limited but interesting role in biology. Although they are not usually found in the primary structure of proteins. But they do have significance in specific biological contexts. Some organisms, such as certain bacteria and archaea, add D-amino acids to their cell walls.
which resists enzymatic degradation by the host organism. D-amino acids have been detected in trace amounts in some peptides and proteins. which potentially contribute to various biochemical processes including neurotransmission and antimicrobial defense mechanisms. Although their presence is less common than that of L-amino acids.
However, the presence of D-amino acids underscores the complexity and adaptability of biological systems. which demonstrates their importance in specialized functions within the complex web of life. Research on the role of D-amino acids continues to uncover the relevance of various biological pathways and their potential applications in biotechnology and medicine.
What are Left-Handed Amino Acids?
Left-handed amino acids are often referred to as L-amino acids. A class of biomolecules that are the basic building blocks of proteins, one of the essential macromolecules of living organisms. L-Amino acids have a distinct chiral character.
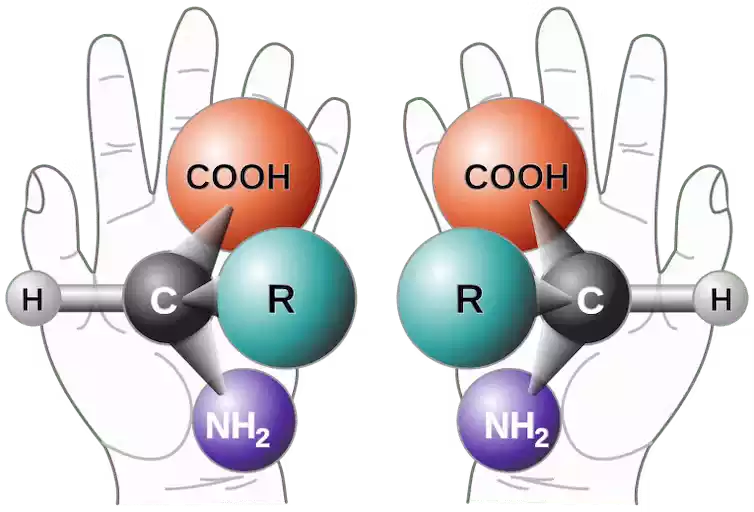
Which means they exist in two mirror-image forms. Their structural elements are asymmetrically arranged. In the context of L-amino acids, this inequality is at the central carbon atom (α-carbon), the amino group (NH2), the carboxyl group (COOH), and the hydrogen atom. And occurs with a specific side chain (R-group). It is connected.
The designation “left-handed” refers to the fact that these amino acids have their amino group on the left while their carboxyl group is on top. The unique chirality of L-amino acids is of utmost importance in biology. Because it ensures the precise folding and three-dimensional structure of proteins. Enables important biological functions to be performed in living organisms.
What are Right-Handed Amino Acids?
Right-handed amino acids, also known as D-amino acids. Biomolecules that share the same basic structure as their left-handed counterparts, L-amino acids, with one important difference. The term “right-handed” refers to the position of their chiral carbon atom (α-carbon). where amino group (NH2), carboxyl group (COOH), hydrogen atom, and side chain (R-group) are asymmetrically arranged.
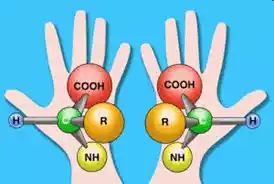
The amino group is positioned to the right while the carboxyl group is at the top. Unlike L-amino acids, D-amino acids are relatively rare in the primary structure of proteins in most living organisms. They play specialized roles in specific biological contexts. Such as cell wall formation in certain bacteria and archaea.
So, they have been detected in trace amounts in various peptides and proteins. indicating their possible involvement in distinct biochemical processes. D-amino acids contribute to the overall structural diversity and complexity of biological systems. Demonstrates their importance in specific biological functions and interesting applications in scientific research.
Amino Acid Structure
Amino acids are organic compounds that serve as the building blocks of proteins.
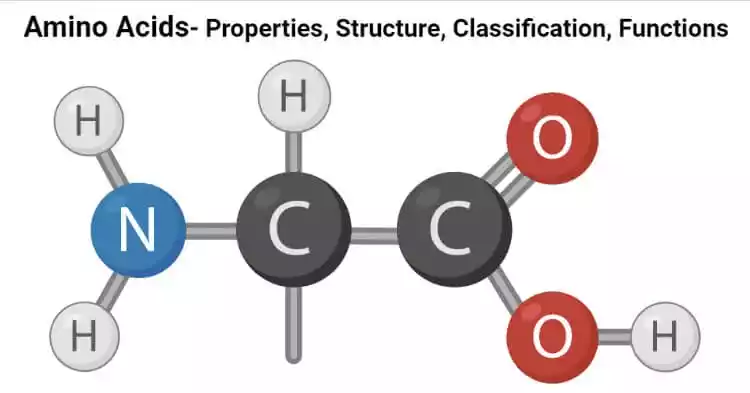
They have a common structure consisting of several key components:
- Amino Group (NH2): This is a functional group containing two nitrogen atoms and a hydrogen atom bonded together. The amino group is attached to the central carbon atom (α-carbon) of the amino acid.
- Carboxyl Group (COOH): The carboxyl group consists of a carbon atom double-bonded to one oxygen atom and single-bonded to another oxygen atom (which is also bonded to a hydrogen atom). It is found at the opposite end of the α-carbon from the amino group.
- Central Carbon (α-Carbon): The central carbon atom serves as the bridge between the amino group and the carboxyl group. It is also bonded to a hydrogen atom and a side chain (R-group).
- Side Chain (R-Group): The side chain, also known as the variable group or lateral chain, is unique to each amino acid. It distinguishes one amino acid from another. The nature of the R-group determines the specific characteristics, properties, and functions of the amino acid. There are 20 common amino acids, each with a different R-group.
Difference Between Left and Right Handed Amino Acids
Here’s a comparison chart highlighting the key differences between left-handed (L-amino acids) and right-handed (D-amino acids) amino acids:
| Characteristic | L-Amino Acids (Left-Handed) | D-Amino Acids (Right-Handed) |
|---|---|---|
| Chirality | Left-handed | Right-handed |
| Orientation of Amino Group | On the left | On the right |
| Occurrence in Proteins | Predominant in proteins | Rarely found in proteins |
| Biological Prevalence | Common in living organisms | Occur in some bacteria and archaea |
| Role in Protein Structure | Contribute to functional protein folding | Limited role in protein structure |
| Function in Cell Walls | Not involved in cell wall formation | Used in cell wall formation by some microorganisms |
| Neurotransmission | Not typically involved in neurotransmission | Some involvement in neurotransmission |
| Abundance in Nature | Abundant in nature | Less abundant in nature |
| Role in Biochemical Processes | Major contributors to diverse biological processes | Involved in specialized functions |
| Pharmaceutical and Biotech Applications | Commonly used in pharmaceuticals and biotech | Limited use in drug development and biotech |
| Analytical Detection Methods | Detected using various analytical methods | Analytical methods similar to L-amino acids |
Chirality in Amino Acids
Chirality in amino acids is a fundamental concept that refers to the property of amino acids having a non-superimposable mirror image or being optically active. Chirality arises from the asymmetric arrangement of atoms around the central carbon atom (α-carbon) in amino acids. This carbon atom is bonded to four different groups: the amino group (NH2), the carboxyl group (COOH), a hydrogen atom (H), and the variable side chain (R-group). It is this tetrahedral arrangement of groups that gives rise to chirality.
Key points about chirality in amino acids:
- Enantiomers: Amino acids come in two enantiomeric forms, which are mirror images of each other but cannot be superimposed, much like a left and right hand. These enantiomers are often referred to as the L-amino acid and D-amino acid forms. The L-amino acids are more commonly found in biological systems.
- Chiral Center: The α-carbon in amino acids is called a chiral center because of its ability to exist in two non-superimposable mirror-image forms.
- L-Amino Acids: In L-amino acids, the amino group is on the left side of the α-carbon when the carboxyl group is at the top. L-amino acids are prevalent in the proteins of living organisms and play a central role in biological processes.
- D-Amino Acids: In D-amino acids, the amino group is on the right side of the α-carbon when the carboxyl group is at the top. D-amino acids are less common in biological systems but are found in some microorganisms and have specialized roles.
- Biological Significance: Chirality is crucial for the function of proteins. The specific arrangement of amino acids in proteins, with all L-amino acids, is essential for proper protein folding, three-dimensional structure, and functionality.
- Racemization: The interconversion of L-amino acids to D-amino acids or vice versa is known as racemization. In living organisms, enzymes control the chirality of amino acids during biosynthesis, ensuring that only the correct enantiomers are incorporated into proteins.
Understanding chirality in amino acids is vital for comprehending the structural and functional diversity of proteins, as well as the unique biological roles of different amino acids in living organisms.
Chirality in Food and Nutrition
Chirality plays a significant role in food and nutrition, particularly in the way our bodies process and interact with certain molecules.
Here are some key aspects of chirality in food and nutrition:
- Amino Acids and Proteins: Chirality is essential in the context of amino acids, which are the building blocks of proteins. In nature, proteins are made up of L-amino acids, and the chirality of these amino acids is critical for the proper folding and function of proteins in our bodies. Dietary proteins, such as those from meat, dairy, and plant sources, contain L-amino acids, which are used by our bodies to build and repair tissues and perform various metabolic functions.
- Sweetness Perception: Chirality also plays a role in the perception of taste. Some molecules, such as certain artificial sweeteners like aspartame, have chiral centers. The two enantiomers (mirror-image forms) of these molecules can taste quite different from humans. For example, one enantiomer may taste sweet, while the other is tasteless or has a different taste. Understanding the chirality of sweeteners is important in food and beverage industries to optimize taste.
- Drug Interactions: Chirality is relevant in pharmacology and nutrition because many pharmaceutical drugs and nutritional supplements contain chiral molecules. The human body can often metabolize and respond differently to different enantiomers of a drug or nutrient. For example, some drugs may have different potencies or side effects depending on their chirality. Understanding these interactions is crucial for drug development and nutritional science.
- Digestive Enzymes: Our bodies use specific enzymes to break down and absorb nutrients. These enzymes are often highly selective for one enantiomer over the other. For instance, enzymes in the human digestive system typically process L-amino acids more effectively than D-amino acids, which are less commonly found in food.
- Chiral Drugs and Nutrients: Some naturally occurring compounds and synthetic molecules in food and pharmaceuticals may exist in chiral forms. It’s important to consider the biological activity and effects of each enantiomer separately. Regulatory agencies, like the U.S. Food and Drug Administration (FDA), often require testing and labeling to ensure the safety and efficacy of chiral drugs and nutritional supplements.
- Chiral Separation: Analytical techniques such as high-performance liquid chromatography (HPLC) and gas chromatography (GC) are used to separate and quantify chiral compounds in food and pharmaceutical products. These methods are essential for quality control and safety assessments.
Chirality is a fundamental concept in food and nutrition with implications for taste perception, drug interactions, nutrient absorption, and the development of pharmaceuticals and dietary supplements. Understanding the chirality of molecules in these contexts is essential for optimizing health and safety in the food and pharmaceutical industries and for advancing our knowledge of nutrition and biochemistry.
Conclusion
The difference between left and right-handed amino acids is a captivating and essential topic. These molecular mirror images, with their distinct roles in biology and chemistry, continue to intrigue scientists and fuel discoveries in various fields. Whether you’re a budding biologist or simply curious about the wonders of the microscopic world, understanding the nuances of amino acids brings us one step closer to unraveling the mysteries of life.