Chemical and Physical Properties: The properties of all substances can be separated into chemical and physical properties. We can identify different humans by their physical characteristics like height, weight, appearance, hair type, and facial characteristics. Also, all substances possess physical properties based on how they are recognized and used. Chemical properties of substances are the properties that determine the way they interact with different substances, and also how they react after exposure to oxidation and heat. There are many distinctions between the physical and chemical characteristics of the substances, which are discussed in this piece.
- The most common physical properties are observable and quantifiable like the form, the color of the object, its hardness, and density of the material. In contrast chemical properties come to focus only when a substance is placed against another to observe the results or see the way they interact with each with each other. The most important property to consider is flammability which determines how the substance can be stored or handled. substances with flammability are more likely to burn than non-flammable substances. In addition, corrosion is an additional chemical property that a substance has which can be seen in the way they oxidize once they contact water.
- In a general way, it could be certain that the property that alters the chemical characteristics of an ingredient is chemical characteristics, while those that don’t cause any alteration in the chemical character of the substance are physical properties. If you’re trying to sniff a particular substance (odor) it is doing nothing to alter the chemical properties therefore it’s an physical property. Examples of physical properties include density, color melting and freezing points magneticity, flammability, viscosity, and density. In contrast, some examples of chemical properties include reactivity with different materials like water, the pH value of the material as well as the heat generated by burning.
- Chemical and physical properties aid us in understanding the nature and essence of the substance and what is the best usage of it for various situations.
What are physical Properties?
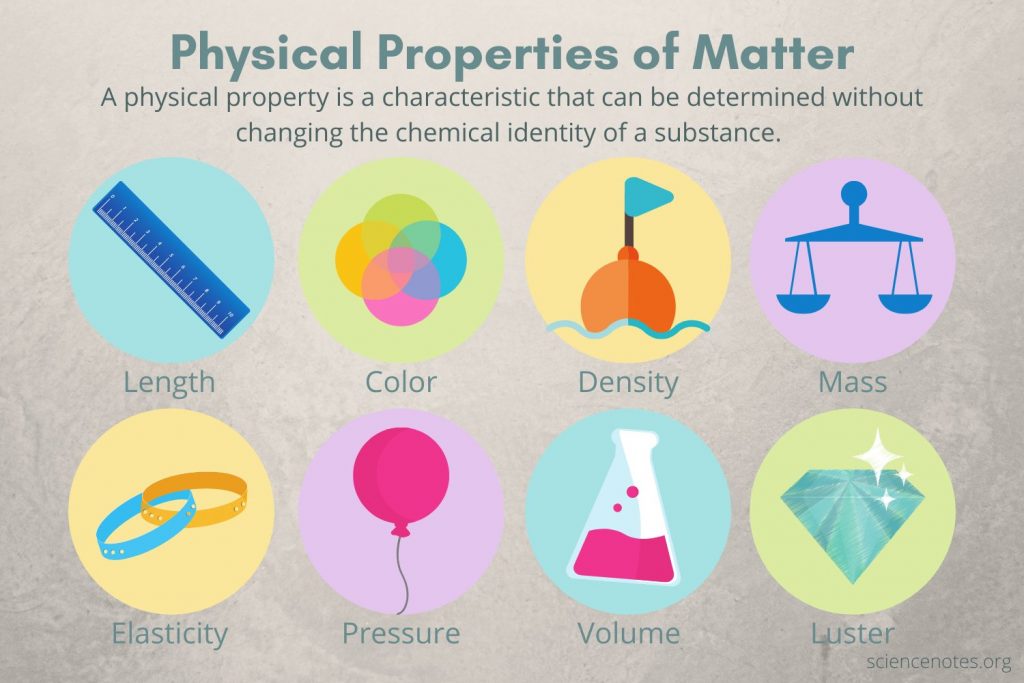
- Physical properties are those which can be measured without changing the chemical composition of the material. The appearance and dimensions of the material with physical properties. No matter the measure used the molecular and chemical nature of the matter remains identical.
- Thus, any property which is able to be measured or observed by conducting any chemical reaction would be classified as a physical property. Furthermore, physical properties are divided into two categories namely expansive properties and intensive properties.
- A property that is intensive is not dependent on the quantity of the substance. The properties that relate to the appearance of a substance are considered to be intensive properties. In particular, color is a property that is intensive since it doesn’t change with the volume of the substance. Similar to the melting point as well as the boiling point of substances as well as density are instances of properties that are intensive.
- The property of extensive properties is based on the quantity of substance. This means that the property of extensive alters when you alter the quantity of the substance. In the case of mass, it is an extensive property because it varies with the quantity of material to be measured. In the same way, length, volume, as well as other dimensions, change as the quantity of matter changes vast characteristics.
What is Chemical Properties?
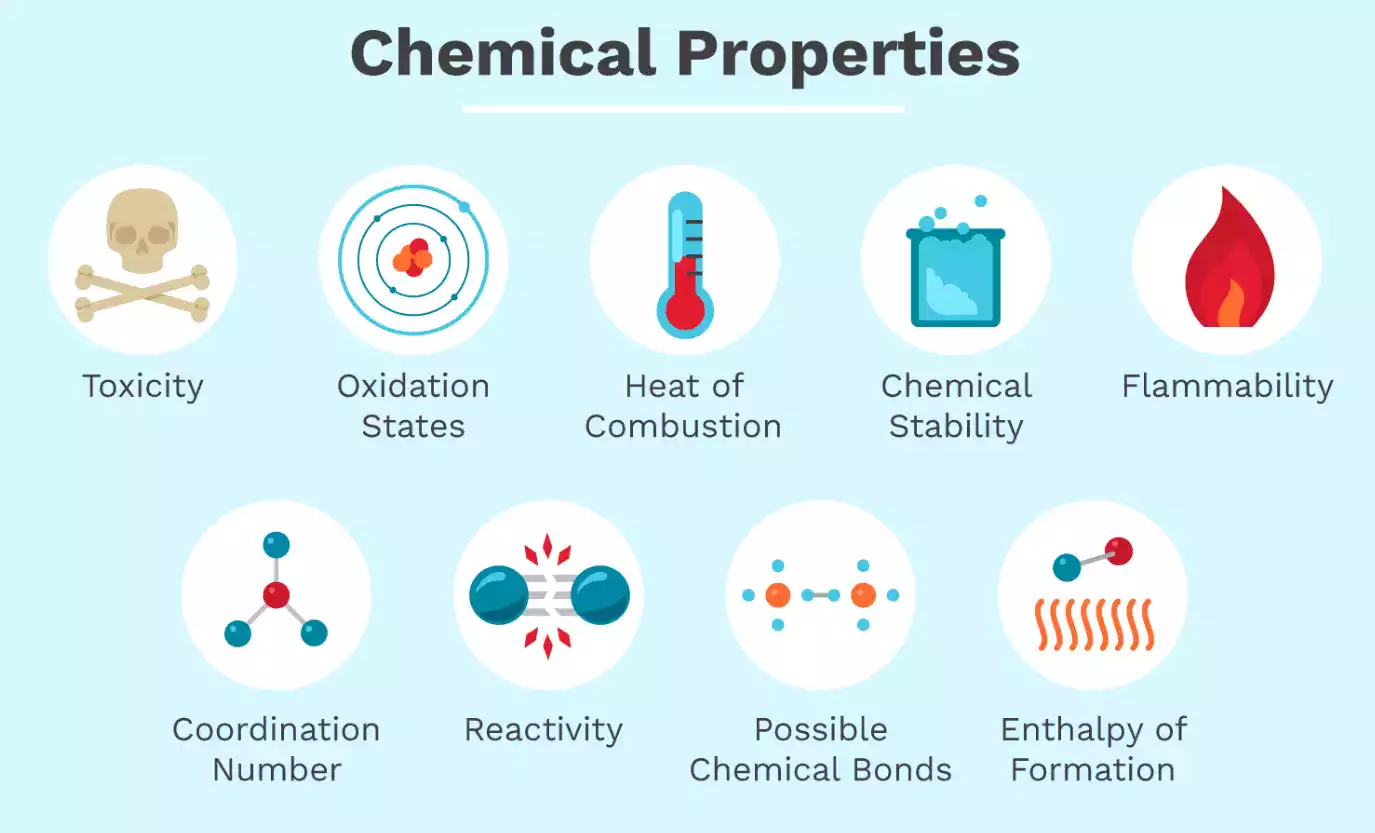
- The definition of a property chemical is that a property’s measurement causes changes within the structure and chemical properties of the material. A chemical property in the substance is evident when there is a chemical reaction or change within the substance. Additionally, a chemical property demonstrates the capability for a substance to mix with other substances, or to transform into another item.
- The chemical composition of an ingredient is also a characteristic that the substance has; when changing its chemical composition the substance changes into a new substance. Chemical properties can be used to measure the transformations a substance goes through during the process of chemical reactions. When the chemical reaction happens it transforms the substance into a completely different substance.
- A chemical property refers to an attribute that is identified in the event of a change within the chemical nature of the material. Examples of chemical properties include toxicity, thermal energy of combustion, chemical reactivity, enthalpy to form as well as oxidation states and radioactivity, etc.
The Table difference between Chemical and Physical Properties:
| Physical Properties | Chemical Properties |
|---|---|
| The chemical identity remains the same, i.e. boiling point of water which means when water boils and gets converted to a vapor state, the chemical identity remains the same. Therefore Chemical formula of water i.e. H2O remains the same after forming gas. | The chemical identity changes in chemical properties. For example flammability – This is the burning of a paper, when a paper is burned then the substance changes its chemical property and gets converted into ash which has different chemical properties. |
| Without changing the chemical composition of the substance the physical properties of a substance can be measured. | By changing the chemical composition of the substance the chemical properties of a substance can be measured. |
| There is no chemical relationship between the bonds of the substance in the case of physical properties. | There is a chemical relationship between the bonds of the substance in the case of chemical properties. |
| The change that occurs in the physical properties of a substance is reversible. | The change that occurs in the chemical properties of a substance is irreversible. |
| A physical property of a substance may or may not depend on the amount of the substance. | The chemical property of a substance does not depend on the amount of the substance |
Differences Between Physical and Chemical Properties
Definition
- Physical Properties: Physical Properties are those that are measurable without altering the chemical nature of matter.
- Chemical Properties: Chemical properties are those that are measured by altering the chemical composition an ingredient.
Chemical Composition
- Physical Property: The physical properties may be assessed without altering the nature of a substance.
- Chemical Properties: Chemical properties can be assessed by altering the nature of the substance.
Chemical Bonds
- Physical Properties: Physical properties don’t directly relate to the chemical bonds that make up the substance.
- Chemical Property: The chemical properties are directly related to chemical bonds.
The amount of substance
- Physical Properties: Physical properties could or might not depend on the concentration of the substance.
- Chemical Properties: Chemical properties don’t depend on the concentration of the substance.
Examples
- Physical Property: Some examples of physical properties are mass and density, color Volume, color, etc.
- Chemical Property: Some examples of chemical properties are the reactivity of chemical compounds, their oxidation states, and coordination numbers, among others.
Conclusion
Chemical and physical properties reveal the distinct properties of substances. The physical property does not alter or alter the chemical composition of the substance. It is distinct from a chemical reaction. But, the chemical property is revealed through exposure to different chemical substances. This is why this article is about the chemical and physical properties, and how they differ from one another. To learn more about this issue, students can seek assistance from Vedantu instructors. They will be given the whole topic in detail with an example and assistance.

