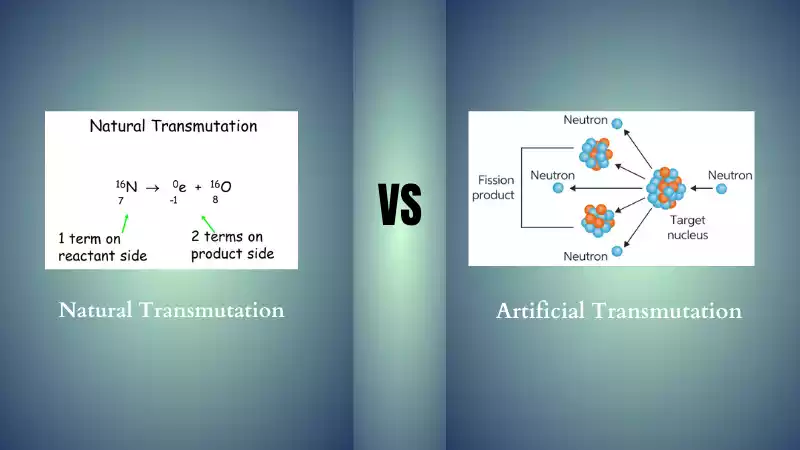
Natural and Artificial Transmutation: Artificial transmutation differs significantly from natural transmutation because its primary form occurs naturally through radioactive decay in stars; while artificial transmutation involves changing an element into another through artificial means.
Transmutation refers to any process which involves altering the nuclei of atoms, leading to their transformation from one chemical element to another chemical element. There are two kinds of transmutation; synthetic and natural.
A brief explanation of transmutation
- Transmutation is the practice of changing one element into another by altering its nucleus by increasing or decreasing protons within its nucleus, creating nuclear reactions between elements that transform one into the other through natural radioactive decay or particle accelerators, or various nuclear procedures.
- Transmutation, the natural process by which unstable isotopes disintegrate over time by emitting radiation and then changing into various elements, plays an integral part in geology, nuclear physics, and astrophysics - among many other disciplines - playing an essential role.
- Artificial transmutation involves stimulating nuclear reactions in order to generate specific isotopes or investigate their properties. Scientists can use high-energy particles such as radio waves to bombard nuclei with high-energy particles that cause them to absorb or release particles before being transformed through artificial transmutation processes.
- Transmutation plays an essential role in numerous scientific disciplines, from biomedicine and energy production to materials science research and the structure of matter studies. Understanding the differences between natural and artificial forms of transmutation is vital to expanding our understanding of nuclear and atomic processes as well as their practical applications.
Importance of understanding the natural and artificial transmutation
Distinguishing between natural and artificial transformations is of vital importance for many reasons:
- Scientific Research: Distinguishing between artificial and natural transmutation allows researchers to distinguish nature-generated processes that occur naturally from those initiated with human influence, providing greater clarity when understanding experiment results, designing experiments, or expanding our knowledge about nuclear physics, chemistry, or any related areas.
- Nuclear Energy and Radioactive Waste Management: Distinguishing between artificial and natural transmutation processes is key in both nuclear energy generation and waste management environments. Natural processes like radioactive decay play an integral part in creating radioactive elements; artificial transmutation techniques using particle accelerators may be employed to alter radioactive waste or convert longer-lived isotopes into shorter-lived versions with lower environmental impacts and storage requirements.
- Medical Applications: Understanding the difference between artificial and natural transmutation methods is vitally important for medical applications, especially radiation treatment and imaging. Isotopes used in cancer diagnosis or therapy may require special consideration due to artificial transmutation methods; thus medical researchers need to know which ones they're dealing with for targeted medical solutions.
- Materials Science and Industry: Understanding artificial and natural transmutation processes is crucial in materials science and industry. Artificial transmutation techniques produce specific isotopes used in various applications, including radioactive tracer and isotopic label production for study purposes, or changing properties of materials. Understanding this difference is vital in optimizing processes and selecting suitable techniques that suit specific industrial or material requirements.
- Risk Evaluation and Safety: Establishing the distinction between artificial and natural transmutation is vital to accurately evaluate the security and risks that might accompany nuclear processes. Researchers and policymakers alike can use it to study transmutation processes as well as their potential effects on the environment and human health and monitor them so as to reduce any possible harm from artificial transmutation processes.
Knowledge of natural and artificial transmutation processes improves our knowledge of fundamental nuclear processes while aiding the creation of new technologies that facilitate the safe and efficient utilization of nuclear energy for medical, scientific, and industrial uses.
Natural Transmutation Details
Natural transmutation is the process by which certain isotopes undergo nuclear reactions that transform them into new elements without human interference, occurring naturally across various environments caused by radioactive decay.
Radioactive decay is the fundamental process by which unstable isotopes known as radionuclides disintegrate into energy in the form of beta particles, alpha particles or even gamma radiation, producing beta particles or alpha particles as by-products and eventually becoming radioactive again. When radioactive decay takes place its unstable isotope becomes more stable through changing composition; eventually becoming radioactive again itself.
There are various natural transmutation reactions that occur with various isotopes. Common examples include:
- Alpha decay occurs when an unstable nucleus of an atom emits an unstable nuclear particle called alpha consisting of two neutrons and two protons, decreasing by two the total number of atoms present and weight by This phenomenon is commonly observed with heavy elements like plutonium and uranium.
- Beta decay refers to the release of beta particle particles from within a nucleus, typically either electrons (beta-minus decay) or positrons (beta+ decay). Beta-minus decay occurs when neutrons transform into protons that emit electrons and antineutrinos; while in beta+ decay the proton gets converted into neutrons which emit proton and neutrino particles simultaneously thereby altering both its atomic number and mass number simultaneously. These processes have the effect of altering both elements while keeping mass numbers constant despite alteration.
- Gamma decay occurs as part of an emission process known as Gamma radiation production, in which high-energy light particles from excited nuclei emit high-energy light particles known as Gamma radiation into space. Gamma decay typically follows alpha or beta decay to further stabilize an unstable nucleus atom; its release does not alter mass or atomic quantity but serves to release any excess energy reserves that accumulate.
- Transmutation processes play a central role in the natural decay of isotopes found in minerals, rocks, and environmental sources, such as geology. Astrophysics also relies on transmutation processes as an analysis tool. A better understanding of their nature allows scientists to date rocks more precisely, study development both on Earth and throughout space-time as well as investigate the behavior of radioactive elements under various settings.
- Natural transmutation processes may compromise the safety of radiation and nuclear energy production. Radioactive decay plays an essential role in nuclear reactor heat generation as well as in producing fuel for the production of nuclear weapons. Furthermore, its radioactive decay helps assess health hazards posed by exposure to radiation as well as ensure proper handling and disposal of radioactive materials.
Natural transmutation mechanisms offer important insight into the behavior of nuclei in their atomic form, the stability of isotopes, and the various natural phenomena that contribute to our world.
Artificial Transmutation Details
Artificial transmutation, also referred to as induced transmutation, refers to the intentional manipulation of nuclear nuclei for the purpose of creating new isotopes or elements via nuclear reactions. As opposed to natural transmutation that takes place naturally over time, artificial transmutation requires human involvement as well as energy sources such as particle accelerators or nuclear reactors as energy sources.
Artificial transmutation involves bombarding target nuclei with high-energy particles like protons, neutrons, or other particle types of atoms - providing enough kinetic energy to offset electrostatic repulsion between charged particles in an atomic nucleus and allow nuclear reactions to take place.
There are several methods and techniques employed in artificial transmutation:
- Particle Accelerators: Particle accelerators are used to accelerate charged particles at high speeds and collide them with nuclei of targets at different energies, producing nuclear reactions which transform target nuclei into desired elements or isotopes. Scientists can regulate both energy usage and particle type so as to induce desired nuclear reactions that create elements or isotopes. Particle accelerators include linear accelerators (linacs) or circular accelerators such as cyclotrons and synchrotrons for this task.
- Neutron Capture: Neutron Capture (Nccap) involves bombarding target nuclei with neutrons generated from sources or reactors, in order to create new isotopes through this process of neutron capture. This method has wide applications in industrial, medical, and research settings alike.
- Radioactive Decay: Transmutation of radioactive matter can also be accomplished using radioactive isotopes. By selecting a parent isotope with specific decay modes (beta or alpha decay), scientists can create their desired daughter isotopes using this process - including creating radiopharmaceuticals for medical imaging as well as therapies.
- Artificial transmutation can be utilized for various applications in multiple fields:
- Nuclear Energy: Artificial transmutation plays an integral part in producing nuclear energy. By bombarding certain isotopes with neutrons and electrons, fissile isotopes like plutonium-239 can be created which are then used as fuel for nuclear reactors. Transmutation reactions also serve to decrease radioactive waste production from reactors.
- Producing Isotopes: Artificial transmutation techniques can be utilized to produce specific isotopes for various uses, including cancer diagnostics and imaging, industrial testing and analysis as well as research purposes. Artificially produced isotopes have many different applications including medical use.
- Fundamental Research: Artificial Transmutation research is vital to our understanding of nuclear and atomic Physics. By studying transmutation reactions, researchers can gain valuable insights into characteristics and behaviors of nuclei within the atomic realm; examine dynamic systems like nuclear power plants; as well as gain an insight into fundamental forces that drive nuclear interactions and forces within nuclei.
Artificial transmutation technologies continue to advance, leading to advances in nuclear science, technology, and their applications. Artificial transmutation techniques help us manipulate nuclear nuclei, create new isotopes and gain greater insight into fundamental components of matter.
Difference Between Natural and Artificial Transmutation
Recognizing the differences between artificial and natural transmutation processes is crucial in understanding their process, mechanisms, and applications. Recognizing these particular characteristics helps clarify what's involved with transmutation processes and their applications.
Here are the primary distinctions:
- Origin: At its heart lies natural transmutation which occurs on its own without human interference and is caused by the radioactive decay of some isotopes. On the other hand, artificial transmutation may also be achieved via nuclear reactors and particle accelerators among other means.
- Occurrence: Natural transmutation is a natural process observed in many situations, such as the radioactive decay of rocks, minerals, and isotopes present throughout Earth's crust. Artificial transmutation on the other hand requires deliberate human action within laboratories or specific technological applications.
- Mechanisms: Natural transmutation can occur through various processes like beta decay, alpha decay, and gamma decay which occur naturally with unstable atoms and result in the transformation of one element to another by altering neutron and proton count in their nuclei.
- Energy Requirements: Natural transmutation does not require external energy input because it occurs naturally. Energy requirements come from radioactive isotopes' inherent instability. By contrast, artificial transformation requires significant amounts of energy inputs such as accelerators or reactors in order to speed up particles, produce neutron flux, or trigger nuclear reactions with nuclear reactions triggered.
- Control and Manipulation: Transmutation is a natural process that cannot be fully manipulated by humans; rather it relies on the intrinsic qualities of decaying isotopes to dictate its course. Artificial transmutation however permits precise control and modification, enabling scientists to focus on particular isotopes for transmutation, regulate reactions accordingly, or create desired elements or isotopes.
- Application: Natural transmutation can have implications in multiple fields such as geology, nuclear physics, and astrophysics; it offers insight into radioactive isotope behavior as well as naturally occurring nuclear reactions. Artificial transmutation on the other hand is used for the production of nuclear energy and producing isotopes for industrial use as well as medical research as well as the fundamental study of nuclear physics and the management of radioactive waste.
- Burstiness: Natural transmutation usually features more unpredictable sentence structures due to its intricate system of decay and nuclear reactions involved; conversely, artificial transmutation could be more uniform due to experimentally designed processes and controlled reactions that take place simultaneously.
Understanding the differences between natural and artificial transmutation processes is essential to furthering scientific advancement, developing applications of nuclear technology, and ensuring safe, responsible use.
Implications and Applications
Knowing the significance and applications of both artificial and natural transmutation are integral components of technological, scientific, and industrial endeavors. Here are some effects and applications of transmutation:
- Nuclear Energy: Transmutation in Nuclear Energy is an integral component of nuclear power production. Artificial transmutation methods are utilized to produce or convert isotopes used to fuel nuclear reactors; through transmuting specific isotopes there may be opportunities to generate energy production while decreasing radioactive waste generation as well as improving safety and efficiency during power production.
- Radioactive Waste Management: Transmutation can offer effective ways of controlling radioactive waste. Through artificial transmutation, highly radioactive isotopes could be converted to less radioactive forms that have shorter lives and reduced radioactivity, making nuclear waste easier and safer to handle over time.
- Isotope Production: Artificial transmutation has long been used as an efficient means to produce isotopes for various applications. Medical isotopes like technetium-99m are essential in medical imaging diagnostics while industrial isotopes may be employed for controlling quality materials analysis and quality testing within industries like petroleum production or agriculture production, or even research applications like nuclear physics experiments, scientific experiments, or fundamental research.
- Materials Science: Transmutation in Materials Science can have serious ramifications for engineering and science alike. Through transmutation techniques, scientists can modify materials' properties and create innovative ones with distinctive characteristics. Transmutation techniques may also help improve properties, increase performance for various applications, and potentially discover the development of new materials to advance technology.
- Fundamental Research: Transmutation reactions provide the chance for fundamental research in nuclear physics as well as related fields, providing scientists with an opportunity to study transmutation reactions as an avenue for basic research in these fields. By studying their effects, scientists are able to gain an insight into nuclear structures, nuclear reactions, nuclei structure, and behavior as well as transmutation experiments' ability to help increase understanding of forces that drive and interact within nuclear nuclei - helping advance nuclear physics as well as related fields.
- Medical Applications: Transmutation plays an essential role in medical applications, with artificially produced isotopes used for imaging applications like Positron Emission Tomography (PET) and single Photon Emission Computed Tomography (SPECT) as diagnostic imaging methods. Transmutation also assists in creating radiopharmaceuticals designed to target cancer treatment, along with therapeutic procedures utilizing radioisotope therapies.
- Environmental and Geological Studies: Natural transmutation processes can be employed for both geological and environmental research. By tracking how isotopes decay naturally, scientists can use radiometric dating techniques to establish the age of minerals, rocks, and archaeological artifacts using techniques like radiometric dating. Natural transmutation also aids researchers in comprehending Earth's geologic history as well as isotopic compositions and environmental processes.
Table Comparison:
| Aspect | Natural Transmutation | Artificial Transmutation |
|---|---|---|
| Definition | Occurs spontaneously in nature | Induced or created by humans |
| Process | Involves radioactive decay or nuclear reactions in natural environments | Involves particle accelerators or reactors |
| Occurrence | Found in stars, supernovae, and radioactive decay of isotopes | Produced in laboratories or nuclear facilities |
| Time Scale | Takes millions to billions of years | Can occur within seconds or minutes |
| Elements Involved | Involves isotopes of naturally occurring elements | Can involve stable or artificial isotopes |
| Applications | Contributes to elemental abundance and radioactive dating | Used in nuclear power, medical imaging, and research |
| Control | Not easily controllable | Can be controlled and manipulated by humans |
| Products | May produce stable or radioactive isotopes | Can produce desired isotopes or elements |
Conclusion
Transmutation, in the end, is an intriguing natural phenomenon and is also induced artificially. Natural transmutation is based on the inherently unstable nature of radioactive elements that causes spontaneous decay as well as transformation. Contrary to this artificial transmutation requires deliberate nuclear reactions that are triggered by external sources. Every type of transmutation comes with its own distinct properties and applications, which contribute to our comprehension of the universe and open the way to new technological and scientific advances.