Organic and Inorganic Nitrogen: Inorganic nitrogen differs from organic nitrogen in that it exists as inorganic compounds. Organic compounds are chemical substances composed primarily of carbon and hydrogen elements; organic nitrogen refers to any amount present within an organic compound. Conversely, inorganic compounds contain elements other than carbon and hydrogen but can still contain both elements, unlike organic compounds which must contain both. Inorganic nitrogen refers to any amount bound up with these inorganic compounds – terms often employed when discussing soil chemistry.
A brief explanation of nitrogen’s importance in biological systems
- Nitrogen plays an essential role in biological systems. As an essential constituent of many biomolecules – such as amino acids and proteins – nitrogen helps form the basis of life and is integral in numerous biochemical reactions that occur within living systems.
- Amino acids form the basis for protein molecules that play an essential role in cell and tissue structure, function, and regulation. Their nitrogen atoms allow for the creation of peptide bonds between amino acids to create functionally specific proteins with unique functions.
- Nitrogen is an integral component of nucleic acid, the storage and transmission medium for genetic information. Each nucleotide in DNA and RNA contains nitrogenous bases which play an essential role in both genetic code encoding as well as protein synthesis and protein protection processes.
- Nitrogen plays an integral part in energy storage and transfer processes, including ATP (adenosine diphosphate) being the primary energy currency within cells. Furthermore, its nitrogen element plays an essential role in producing energy-metabolizing molecules like Urea produced during animal protein metabolism processes.
- Nitrogen plays an integral part in biochemical reactions. As an essential nutrient for plants, its availability impacts plant growth, development, yields, photosynthesis and more. Nitrogen plays an integral part in photosynthesis through its role as an ingredient of enzymes used for catalyzing reactions within cells. Furthermore, nitrogen plays a major role in its presence within plant tissues for photosynthesis processes to take place successfully.
- Nitrogen’s significance to biological systems can be seen in its structural, functional, and regulatory functions in organisms as well as genetic information storage in ecosystems and productivity of ecosystems. Understanding its significance for agriculture, ecology, and biochemistry are also critical fields.
Organic Nitrogen Details
Organic nitrogen, or carbon-based molecules with hydrogen and oxygen atoms attached, can be found in living organisms or made up of recycled organic matter from waste materials, and are crucial components in biological processes.
Definition and Properties of Organic Nitrogen:
- By “organic nitrogen”, we refer to any nitrogen atoms present in organic compounds.
- Nitrogen-containing organic compounds include an array of substances such as amino acids and proteins as well as nucleic acid, urea and uric acids.
- Organic nitrogen compounds often feature covalent bonds between nitrogen and other elements such as hydrogen, carbon, oxygen and sometimes sulfur.
Common sources of organic nitrogen:
- Living organisms, such as animals, plants, and microorganisms produce organic nitrogen compounds.
- Organic matter such as decaying plant materials and animal waste, organic fertilizers, and waste products contain high concentrations of nitrogen-rich organic compounds.
Examples of organic nitrogen compounds:
- Amino acids form the building blocks for proteins. Each amino acid possesses nitrogen-containing amino groups (-NH2).
- Proteins are complex molecules composed of chains of amino acids. They play various roles within tissues, cells, and organisms.
- DNA and RNA nucleic acids both consist of nitrogenous bases such as adenine (adenine), cytosine (cytosine), guanine (guanine), and thymine/uracil.
- Waste products are created through animal protein metabolism.
Role of organic nitrogen in biological processes:
- Organic nitrogen compounds form the building blocks for proteins, nucleic acids, and enzymes.
- Proteins play a variety of functions. They can accelerate biochemical reactions, provide structural support, and transport molecules as well as act as antibodies.
- Nucleic acids provide vital genetic information essential for inheritance and protein synthesis.
- Urea and uric acids are waste products that help remove excess nitrogen from animals’ bodies.
- Understanding the role of organic nitrogen is fundamental for biochemistry, agriculture, and nutrition as well as environmental science. Understanding its function enables us to gain an in-depth knowledge of processes like growth and development, metabolism, and nutrient cycling among living organisms.
Inorganic Nitrogen Details
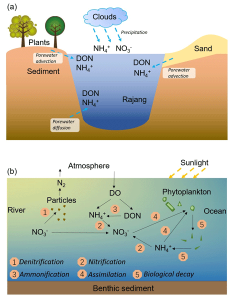
Inorganic nitrogen refers to any form of nitrogen not bound to carbon or derived from living organisms. Inorganic nitrogen plays an integral role in many biological and environmental processes including energy transfer and recycling of nutrients. Inorganic nitrogen plays an integral role in many environmental and biological processes as it provides energy transfer as well as the recycling of nutrients through biological systems. Its use is essential in many processes related to energy transfer as well as the recycling of resources through biological systems.
Definition and characteristics:
- Inorganic nitrogen refers to any form of nitrogen that does not occur as organic molecules but instead occurs as mineral compounds or ions.
- Inorganic nitrogen compounds include both nitrogen gases (N2) and inorganic ions such as nitrites (NO2-), nitrates (NO3+), ammonia (NH3), and ammoniums (NH4+).
Common sources of inorganic nitrate:
- Most organisms cannot directly use atmospheric nitrogen (N2), a gas found primarily in Earth’s atmosphere.
- Plants can absorb nitrate (NO3+) and nitrite(NO2-) ions found in soil, water, and fertilizers.
- Ammonia can be released by organic matter decomposition while ammonium ions can be created through chemical conversion processes or biological mechanisms, such as nitrogen fixation.
Examples of inorganic nitrogen compounds:
- Ammonium Nitrate is an ammonium and nitrogen fertilizer commonly used by plants to easily absorb their nitrogen supply.
- Nitric acid, produced through the oxidation of nitrogen dioxide (NO2), is a strong acid commonly used as both laboratory and industrial reagents.
- Nitrogen gas is the predominant component in our atmosphere and is inert to life; most organisms cannot utilize its nutrients directly for survival.
Role of inorganic nitrogen in biological processes:
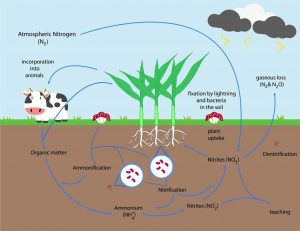
- Nitrogen fixation refers to the process by which certain bacteria and archaea convert atmospheric nitrogen (N2) into ammonia (NH3) for use by other organisms.
- Nitrification is the process by which bacteria convert ammonia into nitrite and eventually into nitrate, leading to further processes in which ammonia can be converted to other chemicals like nitrite.
- Denitrifying bacteria transform nitrate gas (NO3 -) back into nitrogen gas (N2) to complete the nitrogen cycle and return its essential element back into the environment.
- Inorganic nitrogen compounds are essential nutrients for plants. They play an integral part in the biosynthesis and production of amino acids and proteins.
- Understanding how nitrogen transforms in ecosystems and its importance in managing agricultural systems is vital for assessing water quality, studying ecological processes, optimizing fertilizer usage, and avoiding pollution while keeping the nitrogen cycle working in both natural and anthropogenic environments.
Table Differences:
| Aspect | Organic Nitrogen | Inorganic Nitrogen |
|---|---|---|
| Chemical composition | Contains carbon-nitrogen bonds | Does not contain carbon-nitrogen bonds |
| Examples | Amino acids, proteins, nucleic acids, urea | Nitrogen gas, nitrate, nitrite, ammonia |
| Source | Derived from living organisms or remains | Atmospheric nitrogen, mineral compounds, ions |
| Biological utilization | Building blocks of biomolecules | Nutrient cycling, enzymatic reactions |
| Availability | Requires decomposition and conversion | Readily available for uptake and utilization |
| Nutrient release | Slow-release of nutrients | Rapid release of nutrients |
| Environmental impact | Can contribute to eutrophication | Can lead to nutrient pollution and runoff |
| Conversion to other forms | Converted to inorganic nitrogen through decomposition and mineralization | Can be assimilated into organic compounds through uptake and metabolic processes |
| Impact on water bodies | Can contribute to algal blooms and oxygen depletion | Can contaminate groundwater and cause eutrophication |
| Agricultural use | Found in organic fertilizers | Used in inorganic fertilizers for rapid plant growth |
OrganicNitrogen VS Inorganic Nitrogen
There are various methods available to us for categorizing inorganic and organic nitrogen compounds.
Here are a few key differences:
Chemical Composition:
- Organic nitrogen: Organic nitrogen compounds contain covalently bound nitrogen atoms with carbon as well as elements such as hydrogen, oxygen and sulfur.
- Inorganic nitrogen: These are noncarbon-nitrogen compounds and can be found as minerals or ions.
Source and availability:
- Organic nitrogen comes from living organisms and their remains; sources include decomposing animal and plant material, waste products, and organic fertilizers. Its abundance is vast.
- Inorganic nitrogen refers to any source other than atmospheric nitrogen (N2), including sources such as nitrites (NO3-), ammonia (NH3 and NH4+), or nitrates (NO3+). Such forms of inorganic nitrogen can be found in soil, water, or industrial processes.
Biological utilization and transformation:
- Organic nitrogen: Organic nitrogen is essential in the biosynthesis of proteins, nucleic acids, enzymes, and other biomolecules; its energy can then be converted to heat via enzymatic processes.
- Inorganic Nitrogen: Inorganic compounds of nitrogen play an essential role in ecosystems by providing essential nutrient cycles. Nitrogen fixation involves the process of converting atmospheric nitrogen (N2) to forms usable for humans – such as ammonia). Nitrification then converts ammonia into nitrate which plants can absorb; denitrification then completes this cycle back to nitrogen gas, closing out its cycle.
Environmental impact and implications:
- Organic nitrogen: Organic nitrogen is produced through the breakdown of organic matter, providing ecosystems with essential nutrients. If there is too much organic nitrogen present, however, this can lead to eutrophication, leading to harmful algae blooms in aquatic environments as well as oxygen depletion.
- Inorganic Nitrogen: Nitrate and other inorganic nitrogen compounds are vulnerable to runoff and leaching, potentially polluting groundwater supplies and polluting water bodies with nutrients. High inorganic nitrogen levels have adverse impacts on aquatic and terrestrial ecosystems.
Examples of compounds:
- Examples of organic nitrogen compounds: Amino acids and proteins, nucleic acids, urea, and uric acids as well as various organic fertilizers are examples of organic nitrogen compounds.
- Inorganic nitrogen: Inorganic nitrogen compounds include nitrogen gas (N2) and ammonia (NH3); also present are ammonium nitrate, nitric acid, and other compounds which contain nitrogen atoms.
Understanding the difference between organic and inorganic nitrogen is crucial in many fields including biochemistry, agriculture, and environmental studies. Doing so allows users to manage nitrogen cycles more effectively while optimizing fertilizer usage and decreasing environmental impacts.
A Comparison Chart About Organic and Inorganic Nitrogen
| Aspect | Organic Nitrogen | Inorganic Nitrogen |
|---|---|---|
| Chemical Composition | Contains carbon-nitrogen bonds | Does not contain carbon-nitrogen bonds |
| Examples | Amino acids, proteins, nucleic acids, urea | Nitrogen gas, nitrate, nitrite, ammonia |
| Source | Derived from living organisms or remains | Atmospheric nitrogen, mineral compounds, ions |
| Biological Utilization | Building blocks of biomolecules | Nutrient cycling, enzymatic reactions |
| Availability | Requires decomposition and conversion | Readily available for uptake and utilization |
| Environmental Impact | Can contribute to eutrophication | Can lead to nutrient pollution and runoff |
| Conversion Processes | Decomposition, mineralization | Nitrogen fixation, nitrification, denitrification |
| Impact on Water Bodies | Can contribute to algal blooms and oxygen depletion | Can contaminate groundwater and cause eutrophication |
| Agricultural Use | Found in organic fertilizers | Used in inorganic fertilizers for rapid plant growth |
| Interactions with Elements | Can interact with other elements | Can interact with other elements |
What are the sources of organic nitrogen and inorganic nitrogen?
Sources of organic nitrogen:
- Organic matter in the soil: Organic nitrogen is produced through the decomposition of organic materials like animal waste, dead plant material, and soil microorganisms; also included are plant roots, fallen leaves, and decaying organic substances that decay over time.
- Decomposing animal and plant residues: When plants and animals die and their tissues decompose into organic nitrogen pools in the soil, this helps provide new sources of organic nitrogen for crops or livestock manures to use as fertilizer.
- Organic Fertilizers: Organic fertilizers such as manure, compost, and bio-based products contain organic compounds which provide organic nitrogen from natural sources for slow release into the soil.
Sources of Inorganic Nitrate:
- Atmospheric Nitrogen: Our atmosphere contains most of its nitrogen in the form of nitrogen gas (N2). Most organisms cannot directly use atmospheric nitrogen without first going through conversion processes.
- Nitrogen Fixation: Nitrogen-fixing bacteria found in soil are capable of converting atmospheric nitrogen into forms that plants can use, including ammonium (NH4+). This biological process provides ecosystems with inorganic nitrogen sources.
- Synthetic fertilizers: Synthetic fertilizers are produced using industrial processes, providing a readily-accessible source of inorganic nitrogen in modern agriculture. Common examples include ammonium nitrate or ammonium-sulfate fertilizers. They’re readily available to plants as sources of vital inorganic nitrogen.
Organic nitrogen comes primarily from sources found in nature; inorganic sources (atmospheric nitrogen fixation and synthetic fertilizers) can also provide us with access to nitrogen sources.
Similarity:
| Aspect | Organic Nitrogen | Inorganic Nitrogen |
|---|---|---|
| Importance | Crucial for biological systems | Essential for biological systems |
| Role in Proteins | Building blocks and structural elements | Required for protein synthesis |
| Role in DNA/RNA | Component of nucleic acids | Nitrogenous bases in nucleic acids |
| Biological Activity | Involved in enzymatic reactions | Catalysts for biochemical processes |
| Nutrient Function | Essential nutrient for living organisms | Essential nutrient for living organisms |
| Ecological Impact | Can impact nutrient cycles and ecosystems | Can impact nutrient cycles and ecosystems |
| Interactions | Can interact with other elements | Can interact with other elements |
| Metabolic Processes | Participate in energy transfer processes | Participate in energy transfer processes |
| Environmental Impact | Can contribute to eutrophication | Can contribute to eutrophication |
| Role in Agricultural Systems | Support plant growth and productivity | Support plant growth and productivity |
Conclusion
In the end, the two types of nitrogen play a crucial role in helping the growth of plants as well as ensuring productivity. Organic nitrogen can provide long-term, sustainable benefits and less environmental risk, whereas inorganic nitrogen can provide immediate solutions to deficiencies in nutrient levels. The decision between these two kinds of nitrogen is based on the specific requirements of your crop the soil’s conditions and environmental concerns.