Activation Energy and Threshold Energy: The main distinction is that activation energy refers to the potential difference between reactants and activated complexes, while threshold energy refers to the energy required for reactants to collide together and form an activated complex.
Energy is defined as the capacity to perform work. We can harness energy for various tasks; for instance, in chemistry, it could mean chemical or nuclear reactions; here, the terms activation energy and threshold energy are often used to describe two distinct forms of energy.
Definition of Activation Energy
The activation energy is the minimum amount of energy necessary for any chemical reaction to occur. It’s the energy necessary to break apart reactant molecules and form new bonds; ultimately resulting in products. It represents the difference in energy between reactants and transitional states; during reaction, these states often occur as old bonds break and new ones form. Activation energy plays a crucial role in how fast reactions progress; reactions with higher activation values tend to progress more slowly than reactions with lower activation values.
Definition of Threshold Energy
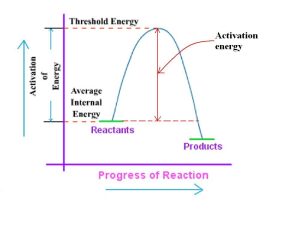
Threshold Energy Please forgive any confusion caused by my answer to your previous question about threshold energy. As it’s rarely used in relation to chemical reactions or physical processes, there is no universally agreed-upon definition for threshold energy in scientific communities.
Threshold energy can be defined broadly as the minimum amount of energy necessary for an event or phenomenon to take place, used widely across disciplines including physics and electronics. This term describes an energy level that enables certain effects or outcomes to become possible or discernible.
Threshold energy refers to the minimum amount of energy necessary to convert a semiconductor device (such as a diode or transistor) from its nonconducting state into its conducting state, or, in photonics terms, for the excitation of an electron inside of the material.
Note that threshold energy’s definition and application can differ depending on its use within different fields or disciplines, so it is crucial to keep an open mind when considering it as part of an approach to studying threshold energy.
Importance of understanding the difference
Learn the difference between threshold energy and activation energy for multiple reasons.
- Conceptual Clarity: Distinguishing activation energy from threshold energy helps to clarify the principles underlying various fields of research. Understanding their meanings and contexts will aid you in avoiding confusion while deepening your knowledge of chemical, physical, and other related processes and phenomena.
- Reaction Kinetics: Activation Energy is a central concept in chemical reaction kinetics, serving to measure reaction rates and reveal mechanisms. Scientists can optimize conditions for chemical reactions by understanding activation energy – including factors like temperature, catalysts, and more.
- Energy Barriers: Activation Energy is the energy hurdle reactant molecules must overcome to reach the transition state and form products. Understanding this barrier will enable you to better predict reaction speed, feasibility, and conditions necessary to surmount it.
- Process Thresholds: To reach desired outcomes in many physical processes, energy thresholds must be passed to achieve certain results. By understanding these thresholds and designing systems with these energy points in mind, scientists and engineers can create reliable and efficient systems. Recognizing threshold energy in electronic devices helps in designing circuits to respond appropriately to input signals.
- Application: Knowledge of threshold energy and activation energy can be applied across numerous fields such as chemical engineering, pharmaceutical research, energy storage systems, and environmental studies. Understanding these concepts allows researchers and practitioners to improve reaction rates, optimize process conditions for processes, create efficient energy systems that provide efficient energy solutions, and effectively address complex challenges.
Understanding the difference between threshold energy and activation energy improves scientific literacy, allows accurate interpretations of phenomena, advances many disciplines and forms the basis of innovation, research, and experimentation in areas where energy plays a central role.
Activation Energy
- The activation energy is the minimum amount of energy necessary for any reaction to take place, and is usually defined as the difference in energy between reactant molecules and transitional states during chemical reactions where old bonds break and new ones form; and between reactants themselves and those that form products during those reactions. As such, activation energy plays an integral part in determining how quickly reactions proceed; reactions with higher activation values tend to progress more slowly than those with lower activation values.
- The collision theory of chemical reaction gives rise to activation. According to this theory, for any chemical reaction to occur, reactant molecules must collide at an appropriate angle and with enough energy; not all collisions of reactant molecules result in a chemical reaction; only collisions equal or exceeding activation energy lead to products being formed.
- Each chemical reaction has a specific activation energy that depends on factors like its reactant, conditions of reaction (temperature), and presence/absence of catalysts. Higher activation energy indicates more energy is required to start it; this slows it down significantly; on the contrary, lower activation energies indicate reactions can take place more rapidly and easily.
Understanding activation energies is important in various fields of chemistry, including chemical engineering, reaction kinetics, and catalysis. Understanding activation energies allows scientists to predict reaction rates more accurately, design efficient reaction conditions and create catalysts with lower activation energy resulting in faster reaction rates. Understanding activation energies also gives scientists insight into whether desired chemical transformations are feasible or not.
Threshold Energy
- My previous answer may have caused any confusion; as the term threshold energy isn’t widely used when discussing chemical or physical reactions and processes. Because there is no universally accepted definition for threshold energy in scientific communities.
- Threshold energy can be loosely defined as the minimum amount of energy necessary to elicit an event or phenomenon and is widely utilized across numerous fields such as physics and electronics. Essentially, threshold energy refers to an energy level that makes certain effects or outcomes possible or noticeable.
- Electronic device makers commonly refer to threshold energy as the minimum amount required to switch a semiconductor device (such as diodes or transistors) from their semiconducting state into conductivity, whereas photonic threshold energy refers to how much photon energy must be expended for specific processes like stimulating electrons within material samples.
Note that threshold energy’s definition and application may differ depending on its context or field of study, making it essential to keep in mind which discipline or domain it will be applied in.
Differences Between Activation Energy and Threshold Energy
There are various distinctions between activation and threshold energy that have their own definitions and applications, as well as key distinctions that exist. Here are a few highlights between threshold and activation energy:
Definition and Scope
Activation Energy refers to the minimum amount of energy necessary for a chemical reaction. It represents the minimum energy required to spark such reactions.
- Threshold Energy: Threshold energy refers to the minimal energy required for an event or phenomenon to take place and can be applied across many processes such as electronic transitions, particle excitation, or triggering specific effects.
- Context: Activation Energy: This term refers to activation energy used in chemical reactions and reaction dynamics to measure rates, influence mechanisms involved, and identify energy barriers.
- Threshold Energy: Threshold energy can be found across numerous fields such as physics and electronics. It refers to processes in which specific energy levels must be exceeded to reach an end result or achieve the desired effect.
Conceptual Variation
- Activation Energy: The activation energy measures the difference in energy between reactants and transitional states during chemical reactions. It quantifies how much energy is necessary to break bonds before creating new ones.
- Threshold Energy: Threshold energy refers to the minimum amount of energy necessary to initiate or observe a specific process or event, serving as an indication of energy required for action or transition.
Impact Analysis Processes and Procedures.
- Activation Energy: The activation energy has an immense effect on both the reaction rate and feasibility of chemical reactions, with higher activation energies leading to slower reactions while lower ones allow faster ones.
- Threshold Energy: A threshold energy level is the amount of energy that determines whether a process will start, take place, or stop altogether. Without sufficient energy available, processes may cease or remain dormant; to reach desired effects or phenomena you must cross over this threshold energy threshold level.
Understanding the differences between activation energy and threshold energy can help accurately describe and analyze processes in their specific contexts.
Relationship Between Activation Energy and Threshold Energy
While activation energy and threshold energy may seem dissimilar, they do have some shared characteristics.
- The activation energy is defined as the minimum amount of energy necessary to initiate a chemical reaction and transform reactant molecules into products. Each chemical reaction’s activation energy is unique and determines how rapidly its progress occurs.
- Threshold energy refers to the minimum amount of energy necessary for an event or phenomenon to take place and can be applied in various ways such as electronic transitions, particle excitation, and activating specific effects.
- Thresholds can be applied broadly across many processes and phenomena. Threshold Energy refers to the energy required to start an event or process and observe it successfully.
- In certain situations, activation energy can be seen as a threshold energy. It represents the threshold that must be exceeded for chemical reactions to progress further and is also the minimum amount of energy necessary to overcome an energy barrier and reach transitional states where reactant molecules undergo bond-breaking/formation processes.
- Remember that threshold energy is more of an abstract concept used to characterize many processes, including chemical reactions. On the other hand, activation energy refers specifically to barriers present during chemical reactions.
While activation energy can be applied to chemical reactions, threshold energies represent a more general concept. An activation energy barrier exists when applied specifically to chemical reactions while threshold energies refer to any minimum amount of energy necessary to trigger certain events or phenomena in many different processes.
Conclusion
The concept of threshold energy and activation energy are two fundamental concepts that support physical processes and chemical reactions. While activation energy is specifically related for chemical processes, the threshold can be applied to many phenomena in physics. Both play a crucial role for their specific fields, and are able to be applied to a variety of practical applications. Knowing these concepts allows engineers and scientists to come up with creative solutions, create effective processes and create advances in different fields.

