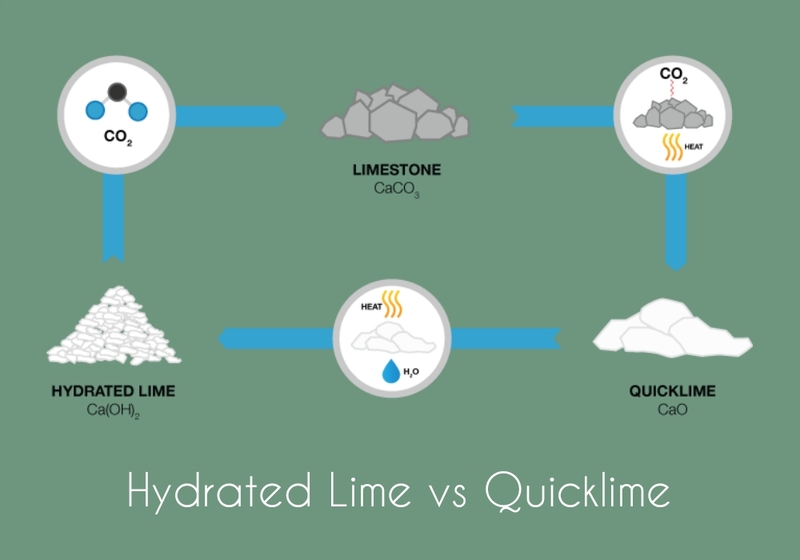
Quicklime and Hydrated Lime: Quicklime, or burnt lime, contains calcium oxide while hydrated lime (slaked limestone) contains calcium hydroxide.
Limestone is the main source of quicklime and Hydrated lime. These compounds, too, are alkaline, just like limestone. Quicklime is also known as “burnt limestone” because it’s produced by thermal decomposition. We call hydrated limestone “slaked” lime because it is produced by quenching the quicklime in water.
Definition of Quicklime

Calcium Oxide, more commonly known as Quicklime (CaO), is an extremely reactive chemical substance made by the calcination of calcium carbonate or limestone, creating a white solid with an extremely high melting point. A lime kiln uses high temperatures (ranging between 900-1200degC), to cause thermal decomposition which releases carbon dioxide gas that quickly decomposes into quicklime that is then sold either as small lumps or in powder form for sale.
Quicklime is an alkaline substance that reacts violently with water to produce heat and form calcium hydroxide. This exothermic reaction, commonly referred to as hydration, makes quicklime an invaluable asset in many industrial processes; agriculturally speaking it provides essential soil pH levels adjustment and essential plant nutrients. Caution should always be exercised when handling quicklime; exposure can result in serious eye irritation, severe burns, and even eye infection.
Definition of Hydrated Lime

Hydrated lime (also referred to as Calcium hydroxide or CaOH2) is a chemical compound produced through the process of hydration by reacting quicklime with water to produce Calcium hydroxide, leaving behind a white, dry powder with an alkaline taste and no smell. Hydration involves adding water and quicklime together for the chemical reaction that converts quicklime into Calcium hydroxide.
To hydrate quicklime, mix equal parts water with quicklime in an exothermic reaction that absorbs all of its molecules; hydrated lime is then typically used as a fine powder that’s commonly applied directly onto surfaces.
Hydrated lime has many applications across a range of industries. It can help enhance the workability, adhesion and durability of mortars and plasters in construction as well as manufacture lime-sand masonry products such as lime-sand bricks. Hydrated lime can also help remove impurities and adjust pH in water supplies as well as being utilized in agriculture, flue gas desulfurization processes, and sugar refining.
Comparative to quicklime, hydrated lime is less caustic and easier to store and handle, making it simpler for storage and handling purposes. Still, caution must be exercised when handling it as its use can cause irritation of the skin or eyes.
Importance and common applications of Quicklime and Hydrated Lime
Quicklime and hydrated limestone are indispensable materials that have a variety of uses across industries, from chemical modifications to pH adjustments. Furthermore, these essential products contribute significantly to construction projects as well as agricultural work and environmental projects.
Quicklime and hydrated limestone can also be found in use in applications including:
- Construction: Quicklime and hydrated limestone are two components used in the creation of cement, mortar, and plaster products that improve workability, bond strength, and overall durability.
- Water Treatment: Hydrated Lime is widely utilized as a water and wastewater treatment agent. It helps adjust pH levels to lower acidity while simultaneously improving disinfection effectiveness, eliminating impurities such as metallic contaminants from water supplies.
- Steel Manufacturing: Quicklime is used as a fluxing agent during steel production, helping remove impurities such as silica or phosphorus from iron ore during refining stages.
- Chemical Manufacturing: Quicklime is widely utilized as a chemical reactant or precursor in many chemical synthesis processes, including calcium carbide production, calcium cyanamide manufacturing, and calcium hypochlorite formation. Additionally, quicklime acts as an important precursor for many other chemicals that need to be produced or synthesized.
- Paper and Pulp Industry: Quicklime is often used to enhance the bleachability of the pulp as well as the quality and finish of paper products.
- Waste and Sludge Treatment: Quicklime and Hydrated Lime are commonly used as an effective waste and sludge treatment, eliminating organic waste odors while helping improve handling and disposal processes. Lime reduces pathogens while improving waste handling and disposal procedures.
Quicklime and hydrated limestone play an invaluable role in numerous industries, from construction and water treatment, to agriculture processes and chemical manufacturing, as well as chemical formulation. Their versatile qualities and chemical composition make them essential tools.
Table Difference Between Quicklime and Hydrated Lime
| Property/Characteristic | Quicklime | Hydrated Lime |
|---|---|---|
| Reactivity | Highly reactive | Lower reactivity |
| Handling and Storage | Requires precautions | Safer to handle and store |
| Moisture Absorption | Hygroscopic | Reduced moisture absorption |
| Physical Form | Lumps, granules, powder | Fine powder |
| Solubility | Sparingly soluble | More soluble |
| pH Levels | Higher pH | Slightly lower pH |
| Applications | Industrial processes, soil modification | Water treatment, construction, agriculture |
| Safety Considerations | Caustic, handle with caution | Less caustic, safer to handle |
| Cost | Generally more cost-effective | May be more expensive |
Chemical Composition and Production
Quicklime (otherwise known as calcium oxyhydroxide or CaO) has an extremely straightforward chemical composition; it consists of one calcium (Ca) and one oxygen atom, hence its chemical formula CaO.
Calcination is the process used to produce quicklime. Calcination involves thermal decomposition at high temperatures of limestone (calcium carbonate or CaCO3) in order to produce quicklime as an end product of a chemical reaction.
CaCO3 (limestone) + CaO (quicklime) = Carbon Dioxide.
Industrial lime kilns heat limestone to temperatures between 900 and 1200 degrees Celsius (1652 and 2192 degrees Fahrenheit), where its thermal decomposition causes carbon dioxide gas release and formation resulting in quicklime that is then collected, cooled down, collected again for further processing, then sold back onto the market.
Chemical Composition and Manufacturing Process:
- Hydrated lime (also referred to as calcium hydroxide, Ca(OH)2) has more complex chemical characteristics than quicklime. It consists of two oxygen atoms, two hydroxide ions and one calcium atom; its chemical formula is Ca(OH)2.
- Hydrated lime can be produced by adding water to quicklime, which triggers the chemical process known as “hydration”.
- Quicklime (Calcium Oxide), H2O, and Ca(OH2)2 (hydrated lime).
- Hydration occurs when water molecules combine to create calcium hydroxide or hydrated limestone through chemical reactions that release heat as quicklime absorbs and transforms. As a result, fine powdered hydrated lime can be created which is then dried for different uses.
Production of hydrated limestone requires careful oversight. In order to meet desired levels of hydration, an accurate amount of water must be added to Quicklime. Water addition must be precisely managed so as to produce consistently graded hydrated lime products.
Physical Properties
Quicklime in its purest form is a white solid that may contain impurities that give it yellow or grayish tints, depending on how purified it is.
Texture: Quicklime can come in the form of lumps, granules, or fine powder depending on its intended use and manufacturing process.
- Quicklime doesn’t possess an obvious scent.
- Quicklime has an approximate melting point temperature of 2,572deg Celsius (4,662deg Fahrenheit).
Water Solubility: Quicklime only dissipates slowly in water. Calcium hydroxide forms when reacting with it, making the quicklime more soluble and increasing its solubility.
Reactivity: Quicklime reacts strongly with water. When exposed to it, an exothermic reaction takes place that produces heat and leads to calcium hydroxide being formed.
Physical Properties of Hydrated Limes
Appearance: Hydrated lime mixes with water and transforms into a fine white powder that is most frequently sold in powder form.
Texture: Hydrated Lime Powder has a dry, soft consistency similar to fine flour.
Odorless Lime is virtually odorless
- Hydrated lime has no melting point as its components decompose before melting occurs.
- Hydrated lime has a relatively low solubility in water, though its solubility increases with temperature rises. Due to its larger surface area and the presence of hydroxide ions, hydrated lime dissolves more rapidly than quicklime.
- Hydrated lime is less reactive than quick lime. When exposed to water, its reactions occur more slowly, producing less energy output while creating a stable suspension of calcium hydroxide.
Quicklime (also referred to as hydrated lime) and its parent material are alkaline substances that can irritate respiratory systems, skin, and eyes, so when handling such materials it is vitally important that protective gear such as gloves and goggles be worn.
Differences in Properties and Characteristics
Different properties and characteristics of quicklime and hydrated lime:
Reactivity: Quicklime has a high reactivity, producing an exothermic reaction that releases heat while producing calcium hydroxide. By contrast, hydrated lime has lower reactivity as its reaction with water occurs more slowly.
Quicklime is highly caustic and should only be handled and stored with care to avoid possible irritation to the eyes and skin. Hydrated lime, by comparison, is less caustic and easier to store and handle.
Moisture Absorption: Quicklime has an exceptional capacity to absorb moisture from its environment, reacting with atmospheric humidity in ways that have an impactful response that may interfere with handling and quality. Hydrated lime absorbs significantly less moisture.
Solubility: Quicklime dissolves only slightly in the water while hydrated limestone dissolves more readily, while hydrated lime readily dissolves to form a calcium hydroxide solution in its place. Quicklime is more acidic than hydrated lime, producing an alkaline solution when mixed with water, while its pH level may differ slightly – although both solutions still produce alkaline solutions when they combine with each other.
Before choosing between quicklime or hydrated lime, it’s essential that you consider their individual properties and characteristics carefully, taking into account factors like reactivity, handling, and intended use.
Benefits and Limitations
- Quicklime has high reactivity, which makes it suitable for applications requiring rapid chemical reactions.
- Quicklime can be applied across numerous industries, from construction to environmental remediation or chemical manufacturing.
- Quicklime offers more cost-effective solutions for certain applications due to its lower cost than hydrated lime.
- Quicklime can be used in agriculture to enhance soil quality and pH levels, leading to improved plant growth.
Limitations:
Liquefied quicklime can cause eye irritation and burns, so to ensure safety when handling or storing quicklime it should be handled and stored with special caution.
Quicklime presents unique handling challenges due to its causticity, reactivity, and reactive nature. As such, appropriate equipment and storage conditions must be in place.
Moisture Sensitivity: Quicklime absorbs moisture from the atmosphere, which causes its quality and handling properties to deteriorate over time, potentially clumping over time and decreasing reactivity.
- Hydrated Lime:
Benefits and Limitations
Benefits:
- Hydrated lime is less caustic than quicklime, making it easier to use and handle while decreasing the risk of irritation and burns.
- Hydrated Lime comes in powder form, making it much simpler and faster to mix and apply than quicklime lumps or granules.
- Hydrated lime is an inert chemical with low reactivity, making it suitable for controlled and gradual reactions – perfect for applications requiring slower reactions.
- Hydrated lime can be an effective means of treating water as it removes impurities while simultaneously balancing pH levels.
Limitations:
- Hydrated lime may present challenges when used in applications that demand rapid and highly reactive chemistry, including those requiring quick lime.
- Hydrated lime may not be appropriate for some industrial processes that call for quicklime due to its high degree of reactivity.
- Hydrated lime often costs more than quicklime, rendering it less economically viable for certain applications.
When choosing the appropriate lime product, it’s essential that you consider both its benefits and drawbacks – from quicklime to hydrated lime – carefully. Think about factors like safety concerns, handling ease, cost efficiency, and more when making your decision.
Safety Considerations
PPE should always be worn when handling quicklime or hydrated lime, including safety goggles and gloves as well as protective clothing to reduce eye, skin, and respiratory system contact.
- Quicklime and lime hydrated, when handled or processed, can create fine particles of dust that can irritate respiratory systems, so use respiratory protection and ensure adequate ventilation as required.
- Quicklime or hydrated lime is an alkaline compound that may cause severe irritation when exposed to skin or eyes, and should always be flushed out using water as soon as possible if in contact. Seek medical assistance as soon as necessary if necessary.
- Storage and Handling Maintain an appropriate climate-controlled storage area when storing quicklime or hydrated lime to avoid moisture absorption, degradation, and potential hazards that may result from its storage. Please refer to our Storage Guidelines for assistance when doing this.
- In case of a spillage, take immediate measures to clean it immediately such as vacuuming and sweeping to minimize dust emissions while clearing away materials that have landed on the floor. Discard these materials according to local regulations.
- Proper packaging should be used to prevent spills and leakage during the transport of lime products, adhering to all relevant regulations in doing so.
- Provide training on the hazards and proper handling procedures associated with quicklime and hydrated lime for personnel handling it.
- Establish and communicate first aid procedures for accidents involving lime products or storage. Provide eyewash stations, first aid kits, and safety showers as appropriate in areas where lime products or storage is being utilized.
When handling quicklime or hydrated lime products, it is vitally important to abide by all manufacturer instructions regarding safety. Furthermore, complying with safety standards and regulations ensures safe handling, storage, and use.
Conclude
In short, quicklime and hydrated lime are two significant limestone derivatives with distinctive characteristics and applications. Quicklime is a highly reactive precursor, coveted due to its high reactivity with water and its application in a variety of industrial processes. However the hydrated lime is a milder and more flexible form that is suitable for applications that require an approach that is less acidic. Knowing the distinctions between these two forms of lime is vital to choose the right compound for specific situations, and ensuring the best results and security in a variety of industries.