Electron Pair Geometry and Molecular Geometry: Geometry in Chemistry refers to how molecules are structured within three-dimensional space. Molecular geometry refers to the arrangement of atoms within a molecule relative to one central atom, while electron geometry refers to how electrons surrounding that central atom behave regardless of bond formation or otherwise. A single couple refers to a pair of electrons not shared among any covalent bonds in which the bond exists; conversely, refers to electrons present within the said bond.
Electron pairs, due to being negatively charged, attract each other, causing electron pairs surrounding an atom’s center point to form as far apart as possible thereby decreasing the force of repulsion. In the presence of one nucleus, its resistance will exceed that of any bond pair affected by two nuclei; this leads to a slight reduction in bond angles.
Molecular geometry and electron geometries of molecules with bond pairs only, such as CH 4 molecules with four bond pairs but no single pairs, correspond precisely. For instance, their molecular geometry, as well as electron geometry, is identical. As an example of this, consider methane molecules; with four bond pairs but no lone pairs that bind carbon’s four electron valence electrons to hydrogen atoms making its molecular structure and electronic geometry identical – its molecular geometry being tetrahedral just like its electronic geometries!
A brief explanation of the importance of understanding molecular structures
Understanding molecular structures is fundamental across various fields of science, from biochemistry and chemistry to archeology and geology. Understanding molecular structures enables scientists to gain valuable insights into their behavior, properties, and interactions – these molecules make up everything around us and are the basis of life itself.
Here are just a few reasons why understanding the molecular structure is so essential:
- Chemical Reactions The arrangement of molecules’ atoms determines their interaction with other molecules during chemical reactions, so knowing its molecular structure allows scientists to anticipate the results of reactions, create novel chemical reactions, and develop more sustainable materials.
- Pharmaceutical Design and Pharmacology In the pharmaceutical industry, understanding molecular structure is critical in creating drugs targeted toward specific enzymes or receptors in the body. Learning how biological systems interact with molecules aids in the creation of safe and effective medications.
- Materials Science Materials can vary considerably in their conductivity, strength, and ability to react depending on their molecular configurations. Understanding their structure enables scientists and engineers to produce high-tech materials tailored specifically for various applications.
- Environmental Studies Understanding molecular structures is critical for understanding environmental processes like atmospheric chemistry and pollutant behavior, and their interactions are also helpful when devising sustainable solutions to environmental problems.
- In biochemistry, understanding the three-dimensional structures of biomolecules like DNA and proteins is crucial in understanding their roles and functions in biological processes – something essential both for biotech research and medical studies.
- With regard to this rapidly developing field of nanotechnology, molecular structures play a critical role in creating nanomaterials with specific properties and use in medicine, electronics, and energy storage applications.
- Predicting Physical Properties Melting points, boiling points, and solubility can all be predicted with molecular structure – an invaluable resource in many industrial processes.
- Catalysis and Industrial Applications Through understanding the molecular structure of catalysts, chemical reactions occurring within different industrial processes can be enhanced leading to greater efficiency and decreased costs.
Understanding molecular structures is at the core of modern chemistry and other sciences, providing invaluable insights for advancements in technologies, medicine materials, and environmental studies that ultimately benefit society and our world as we live it.
Introducing the concepts of electron pair geometry and molecular geometry
Molecular geometry and electron pair geometry are fundamental concepts in Chemistry that enable us to better comprehend how electron pairs are distributed within molecules, as well as their overall form and properties that determine chemical behavior as well as interactions.
Electron Pair Geometry:
- Electron pair geometry refers to arrangements of electron pairs in three dimensions around a central atom in a molecule. It is based on Valence Shell Electron Pair Repulsion (VSEPR), which states that pairs within an atom’s valence shell attract each other; this results in specific geometric configurations which limit this attraction/repulsion behavior, with central atoms usually having more electrons within their valence shell than surrounding ones.
- VSEPR theory considers both bonding and non-bonding electron pairs (lone couples) within an electron, including repulsive forces exerted between them to promote movement at the maximum distance possible – creating various shapes with unique bond angles for electron pair geometries.
Molecular Geometry:
- This concept deals with the three-dimensional shape of molecules by taking into account their location within space as well as any arrangement of their constituent atoms – including bonds as well as isolated pairs – in terms of space. Molecular geometry also refers to how molecules fit within it spatially.
- Molecular geometric single pairs can have a profound effect on the overall form of molecules, taking up much more area than bonds and thus distorting its ideal electron-pair geometry as predicted in VSEPR theory. Their presence may also cause bond angles to differ from their expected values for specific electron-pair geometries.
- Electron pair geometry refers to the study of how electron pair arrangements around an atom follow VSEPR theory rules, while molecular geometry refers to all the atoms comprising a molecule (bonded pairs and single). Understanding both concepts is integral for comprehending molecules’ structure as well as describing chemical characteristics and behaviors; with such knowledge at their fingertips, scientists can make more informed choices in areas like drug design or environmental and material science research.
Mentioning the significance of perplexity and burstiness in writing this article
When writing about electron pair geometry and molecular geometry differences, perplexity and speed are vital. Both elements help keep readers engaged by translating complex scientific concepts in an engaging manner – this combination of confusion and frenzy being essential components to its success.
Perplexity for Engaging Complexity:
- Here, it helps present complex scientific ideas clearly and concisely. Electron pair geometry as well as molecular geometrics require complex concepts from chemistry that may be difficult for readers with different levels of understanding in this area.
- To keep readers engaged and prevent confusion, the text must strike a balance between providing in-depth explanations while also avoiding technical terminology. Employing concise language will allow readers to grasp basic concepts without becoming disengaged or losing interest.
Burstiness for Diverse Interests:
- Burstiness refers to variations in the length and structure of sentences, and in this article including bursts is essential to keeping readers entertained and preventing monotony. Without bursts, readers might become bored quickly when dealing with complex research subjects that need constant focus from readers.
- Utilizing bursts by combining longer, more complex sentences with short, simple ones creates a dynamic flow which makes reading more engaging and enjoyable. Furthermore, making use of examples, bullet points, or analogies increases information flow to make the text more appealing to a wider readership.
- Overall, confusion and excitement work to achieve an ideal balance in the text between providing detailed explanations to make understanding easier for readers, while keeping sentences structured differently for maximum reader interest. By striking this balance successfully, the article effectively conveys electron pair geometry as well as molecular geometry concepts across diverse backgrounds while drawing readers further into the molecular world and its impact on various scientific disciplines.
Electron Pair Geometry
Electron Pair Geometry, commonly referred to as molecular or electron domain geometry, refers to the three-dimensional arrangement of electron pairs surrounding a central atom in molecules. This concept derives from the Valence Shell Electron Pair Repulsion theory which provides a tool to predict order and predictability within molecules.
The VSEPR theory proposes that electrons in an atom’s valence shells are attracted by their negative charges, leading them to pair off to form as far apart an arrangement as possible and achieve equilibrium. This results in various geometric patterns surrounding their central electron that play an integral part in shaping the overall form and properties of molecules.
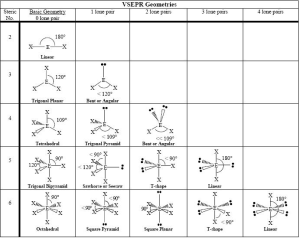
VSEPR theory yields various regular electron pair geometries based on how many electron pairs surround an atom’s center point:
- Linear Geometry occurs whenever there exist two pairs of electrons around an atom’s central point, with bond angles at approximately 180 degrees.
- Trigonal Planar Geometry occurs whenever there are three electron pairs surrounding an atom’s center and their bond angles range between 120 degrees and 130 degrees.
- Tetrahedral Geometry occurs whenever there are four electron pairs that surround an atom’s central point, with bond angles between 109.5deg to 109.5deg.
- Trigonal Bipyramidal Geometry occurs when there are five electron pairs surrounding a central Atom, with bond angles that may range between approximately 120 degrees and 90 degrees.
- Octahedral Geometry refers to the geometric configuration created when six pairs of electrons congregate at an atom’s core and form bonds at angles between 90 degrees and 110 degrees.
- Electron pair geometry provides the basis for molecular geometry, which studies all the atoms comprising a molecule and their relationships to one another, including bonding pairs and free atoms. Electron pair geometries focus exclusively on electron pair movement within central atoms while molecular geometry takes into account its entire three-dimensional form.
Chemical scientists who understand electron-pair geometry can gain insight into the spatial arrangement of molecules’ atoms and gain key insight into their behavior, properties, and interactions – providing invaluable knowledge for fields such as chemical design & development as well as material sciences and environmental science – where understanding molecular structures is vital for research advancement and practical applications.
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within molecules in three dimensions. This involves considering all atoms present including single pairs as it describes their locations within space. Molecular geometry plays a pivotal role in determining the chemical and physical properties of molecules and is therefore essential to Chemistry as an academic discipline.
The molecular structure can be affected by many factors, but one that stands out as most significant is how electron pairs are distributed around an atom’s central region. Electronic pairs can be broken down into two categories – bonding pairs and non-bonding pairings, each exerting force against each other that changes the overall shape of molecules.
Molecular geometry must be understood through its relationship to electron pair geometries, specifically VSEPR theory-inspired electron pair geometries. Electron pair geometries provide idealized models of molecular forms predicted by VSEPR theory; however, single pair changes may alter this idealized version and lead to unexpected molecular shapes compared with that predicted by electron pair shapes.
Lone pairs occupy greater space than bonding pairs, limiting molecular shape. Lone pairs may push bonding pairs closer together than predicted by electron pair geometry and result in smaller bond angles than predicted by its geometry – leading to molecules with lone pairs often having different shapes from their electron pair counterparts.
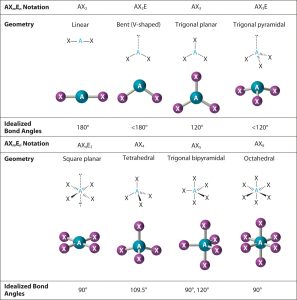
Common molecular geometries emerge through interactions between electron pair geometries and single pair geometries:
Linear Molecular Geometry refers to when a molecule contains two bonding pairs that do not encircle its central atom; all the atoms are arranged along an even line with an angle between them of around 180 degrees.
- Shaped Molecule Geometry refers to an unusual form of molecular geometry that occurs when two bonding pairs and some or all lone pair formation arise around an atom at its center, producing bond angles of less than 180 deg. Bent molecule geometry has become more prominent since recent advances in molecular modeling technology.
- It occurs when a molecule contains three bonding pairs that do not surround any one atom and the arrangement is triangular flat with bond angles between 120 degrees.
- This phenomenon occurs when a molecule has three bonding pairs and one singular pair enclosing its central atom. It resembles the form of an upside-down pyramid and boasts bond angles of less than 120 degrees.
- Tetrahedral Molecular Geometry occurs when a molecule possesses four bonding pairs that all connect directly to one atom and none form single pairs surrounding an atom, creating an arrangement in the shape of a normal Tetrahedron with bond angles of about 109.5 degrees.
- Octahedral geometry occurs when a molecule contains six bonding pairs and there are no single bonds around one atom, placing all atoms in an octahedral arrangement with bond angles of 90 degrees or so.
Understanding molecular geometry is integral for all areas of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. It gives valuable insights into the structure and shape of molecules which directly impacts their chemical behavior, physical properties, and biological roles. Understanding and predicting molecular geometry also plays a vital role in scientific applications like designing drugs, materials synthesis, and environmental studies since it allows scientists to tailor molecules specifically tailored for individual requirements while understanding complex interactions among atoms in compounds’ structures.
Comparison table:
| Aspect | Electron Pair Geometry | Molecular Geometry |
|---|---|---|
| Definition | The three-dimensional arrangement of electron pairs around a central atom in a molecule. | The three-dimensional arrangement of all atoms, including both bonded atoms and lone pairs, in a molecule. |
| Focus | Arrangement of electron pairs around the central atom. | The overall shape of the entire molecule, considering both bonding and non-bonding electron pairs. |
| Factors Influencing Shape | A number of bonding and lone pairs around the central atom, based on the VSEPR theory. | The presence of lone pairs can distort the molecular shape from the predicted electron pair geometry. |
| Prediction vs. Real Structure | Predicts idealized geometric shapes based on VSEPR theory, assuming no influence from lone pairs. | Considers the actual arrangement of atoms, influenced by the presence of lone pairs and deviations from idealized geometry. |
| Bond Angles | Follows specific bond angles for each electron pair geometry (e.g., 120 degrees for trigonal planar). | Lone pairs can cause bond angles to deviate from idealized values, resulting in smaller bond angles compared to electron pair geometry. |
| Applications | Fundamental in predicting molecular shape and properties, impacting fields like drug design and material science. | Crucial for understanding molecular behavior in various applications, such as biochemistry and environmental studies. |
| Steric Effects | Neglects steric effects, which can influence molecular shape in complex molecules. | Consideration of steric effects is necessary to account for deviations in bond angles and molecular shapes. |
| Computational Complexity | Generally simpler to predict based on the number of bonding and lone pairs. | Can be more complex, especially in larger molecules with multiple functional groups and resonance structures. |
| Theory Limitations | Works well for simple molecules, but limitations arise in certain complex species. | May not fully account for electron delocalization and hybridization effects in some molecules. |
Comparing Electron Pair Geometry and Molecular Geometry
Electron pair geometry and molecular geometry are two critical concepts in chemical chemistry for understanding the spatial arrangement of electron pairs and atoms within molecules. While closely related, each concept offers distinct insights that must be taken into account for proper research in chemical chemistry.
Let’s look into them both further:
- Definition: Electron Pair Geometry, also referred to as molecular or electron domain geometry, refers to the three-dimensional arrangement of electron pairs around an atom within molecules. It’s defined using the Valence Shell Electron Pair Repulsion theory which determines how electron pairs align themselves so as to minimize repulsion between themselves.
- Molecular Geometry: In contrast, Molecular Geometry refers to the study of three-dimensional arrangements and configurations of atoms within molecules – including all bonds as well as single pairs. It defines exactly the shape that molecules take in space due to non-bonding electron pairs’ position.
- Electron Pair Geometry: Electron pair geometry’s primary aim is to arrange electron pairs within the central region of an atom’s nucleus in order to form the most ideal geometric form possible by studying their mutual attraction.
Molecular Geometry encompasses all aspects of molecular shape and form, taking into account both electron pair geometry as well as any impact one pair might have on the overall molecular form.
Factors Influencing Shape:
- Electron pair geometry can be determined solely by the bonding and single pair relationships around its central element according to VSEPR theory.
- Molecular Geometry Molecular geometry can be affected not only by its geometrical structure but also by isolated pairs taking up more space than would be idealized when considering electron pair geometry. Isolated pairs take up extra room, distorting their shape.
Prediction and Real Structure:
- Electron pair geometry refers to the ideal arrangement of pairs of electrons according to VSEPR theory, without taking account of any influence from single pairs.
- Molecular Geometry Molecular geometry investigates the exact arrangement of atoms within molecules, including any bonds formed between bonded atoms as well as single pairs that could differ from an ideal electron pair geometry due to repulsion between individual pairs.
Angle Differences:
- When studying electron pair geometry, angles are calculated based on how many electron pairs surround a central atom and their presence is constant for any specific electron pair geometry.
- Molecular Geometry The presence of isolated pairs in molecular geometry may result in bond angles that vary from their idealized values, creating fewer bond angles compared to an equivalent geometric electron-pair geometry.
- Electron pair geometry studies the arrangement of electron pairs within an atom’s core using VSEPR theory, creating idealized shapes using this method. Molecular geometry takes into account non-bonding and bonding electron pairs to determine molecular shape; any deviations of bond angles must also be considered when making decisions about molecular shapes. Both concepts are fundamental for understanding molecular behavior in multiple fields.
Real-World Applications
Understanding electron pair geometry and molecular geometry has numerous practical applications across a number of fields, from drug development to environmental and material sciences research.
Here are a few significant real-world applications of these concepts:
Understanding molecular geometry is fundamental for the design and pharmacology of drugs. By comprehending three-dimensional structures such as those involved with interactions between drug receptors, scientists can develop medications more precisely targeted to specific receptors for increased effectiveness, reduced side effects, and better results from treatment.
- Materials Science and Nanotechnology: The concept of Molecular Geometry is central to materials science and nanotechnology, as it allows materials with specific characteristics to be created through careful consideration of how molecules and atoms are placed together; in the case of semiconductor materials, for instance, exact molecular structures determine conductivity as well as optical properties.
- Molecular geometry can play an essential role in environmental studies: It helps determine how molecules interact with environmental elements like water, air, and soil to influence their fate, transportation, and impacts on both ecosystems and human health.
- Catalysts and Chemical Reactions: Understanding the molecular structures of catalysts is vital for industrial processes. Catalysts serve an integral function in speeding up chemical reactions, with their molecular structures dictating their efficacy; creating more sustainable chemical processes by making catalysts’ shapes more efficient can have great long-term benefits.
- For biochemists: Molecular geometry is an invaluable way of exploring both the structure and function of biomolecules such as enzymes, proteins, and DNA. Understanding their precise molecular structures gives us insight into their biological roles allowing advancements in biotechnology and medicine.
- Molecular: Geometry plays an essential part in crystallography, the science that studies how atoms arrange themselves inside crystals. Molecular Geometry helps us better understand patterns repeating within crystals as well as physical properties which have applications across optics, electronics, and research into materials.
- Green Chemistry: In green chemical chemistry, molecular geometry plays a vital role in creating eco-friendly methods and materials. Being familiar with molecular structure helps chemical engineers come up with alternatives for conventional chemical processes that are eco-friendly.
Limitations and Challenges
Although electron-pair geometry and molecular geometrics are powerful tools for understanding molecules and atoms in space, there are still limitations and issues scientists and researchers must keep in mind when using this approach.
Some major constraints and issues include:
- Simplified Models, Both electron couple geometry and molecular geometrics find their foundations in simplified models like Valence Shell Electron Pair Repulsion (VSEPR). This theory bases its calculations on assuming electron pairs and atoms are rigid spheres, neglecting any more complicated electronic phenomena which might arise within particular molecules; consequently, these models might not adequately depict all aspects of molecular structures involving more complex molecules.
- Steric phenomena occur whenever the weight of molecules or lone pairs causes modifications in molecular geometry that defy predictions made by theory, specifically VSEPR theory. VSEPR theory states that electron pairs will generally oppose each other similarly; however, when coupled with other forces like steric effects this can result in variations to bond angles and shapes – particularly noticeable for larger molecules with many substituents.
- Electron density can become dislocated across multiple atoms in certain molecules with resonance structures, making identification of their geometrical features challenging. This could affect bond lengths and angles making identification of molecular geometry challenging.
- Hybridization of the atomic orbitals can change electron pair geometry and alter the molecular shape. Hybrid orbitals form by mixing standard orbitals to meet molecules’ bonding needs; this leads to deviation from electron pair geometrical shapes.
- Electron pairs that share greater space can cause stronger forces that repel one another than bonds and distort molecular geometry significantly, particularly molecules with single electron pairs in their central sides. This may lead to significant distortions in molecular geometry.
- Gathering accurate experimental data can be difficult when dealing with reactive or unstable molecules, however different experimental techniques like X-ray crystallography or spectroscopy may help identify molecular structures; however, this process takes considerable time and requires special equipment.
- When dealing with massive and complex molecules, accurately predicting their molecular structure becomes increasingly challenging. With multiple functional groups and resonance structures possibly present within its structure, predicting its exact form becomes even harder.
- While VSEPR works well for many simple molecules, it cannot be applied to complexes involving transition metals or polyatomic ions that involve orbital participation; for these situations, alternative theories may be needed.
Even with its challenges and limitations, electron pair geometry as well as molecular geometry remain invaluable tools for understanding molecular structures and predicting molecular behavior. Researchers continue to develop advanced computational methods and experimental methodologies that address their shortcomings while providing a greater understanding of complex molecular geometries in increasingly complicated systems.
Conclusion
Electron-Pair Geometry (EPG), as well as Molecular Geometry (MG), are two key concepts in chemistry, which play complementary roles. The EPG details the arrangement of electron pairs while the MG defines the general molecular structure. Together, they assist us learn about the properties and behavior of various chemical compounds. Through the use of an understanding of the Valence Shell Electron Pair Repulsion (VSEPR) Theory, we can predict molecular structures precisely based on the arrangement of electron pairs. The understanding of EPG and MG has applications across a range of fields of study which makes it a crucial aspect for all chemistry enthusiasts.