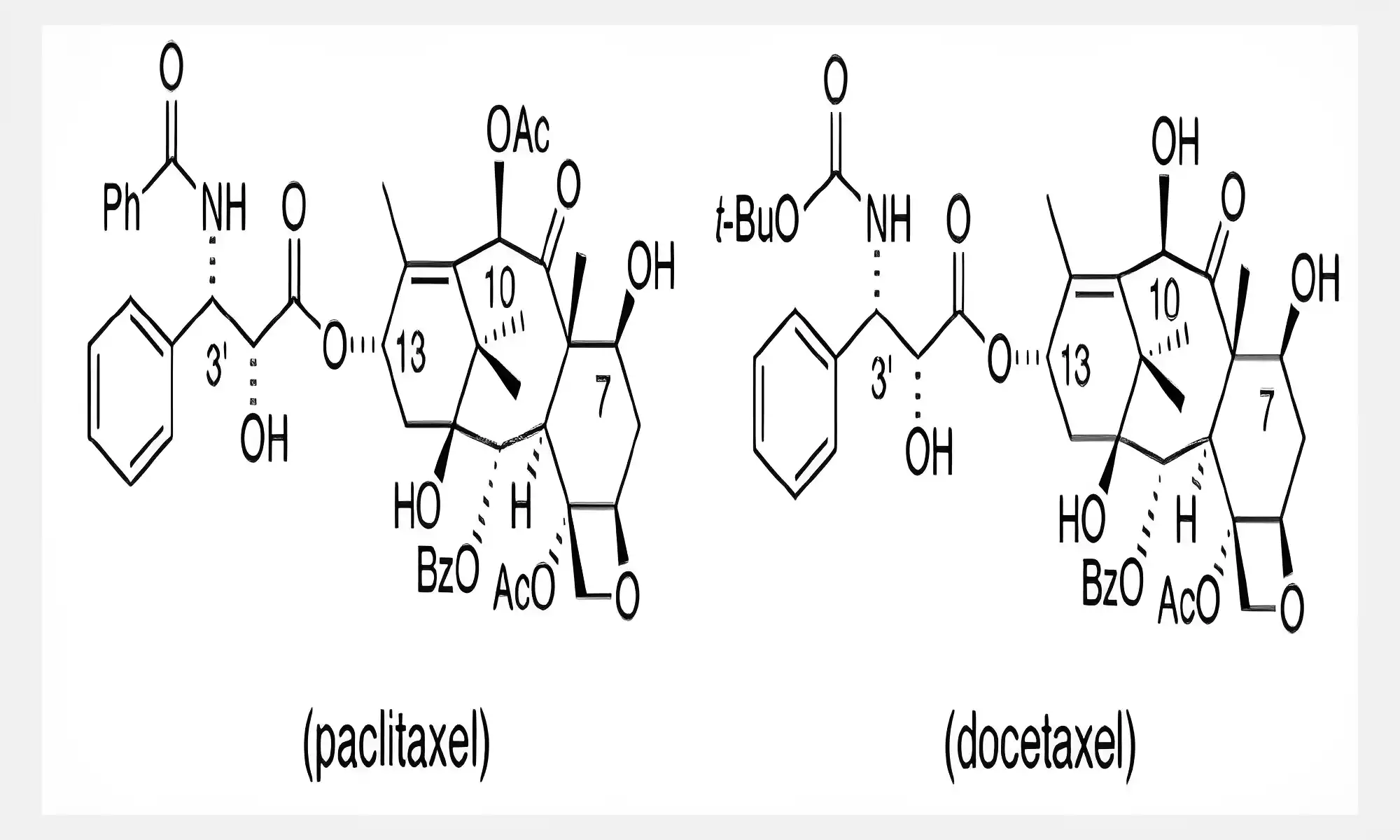
The major distinction between Paclitaxel and Docetaxel is that paclitaxel comes with an ester-side chain in the C13 position, whereas docetaxel has a carbonate tert-butyl ester group in the same point. Taxanes, which include paclitaxel as well as docetaxel are extremely powerful chemotherapeutic medications that are known for their many functions.
Clinical trials conducted in randomized clinical trials have demonstrated their effectiveness as primary-line treatments for ovarian, gastric, lung, and breast cancers that have spread, and are considered adjuvant therapies to treat breast cancer.
It is evident that they have anti-tumor solid capabilities. Docetaxel is a semi-synthetic derivative that was discovered in the 1980s by the European yew, whereas paclitaxel was first discovered in the Pacific yew (Taxus Brevifolia) at the time of 1971.
While the mechanism of action of the different taxanes is comparable, however, they differ in molecular pharmacology, pharmacokinetics, and pharmacodynamic characteristics. These differences could be the reason for the apparent different clinical efficacy as well as toxic profiles of taxanes.
What is Paclitaxel?
Paclitaxel is a chemical therapy that belongs to the taxane class of drugs. It was first taken by the tree’s bark Pacific the yew in 1971. It is manufactured synthetically. Paclitaxel acts by interfering with the microtubule function of cells, which prevents cell division as well as reducing the growth of cancerous cells. It is usually given intravenously to treat a variety of cancers, such as lung,
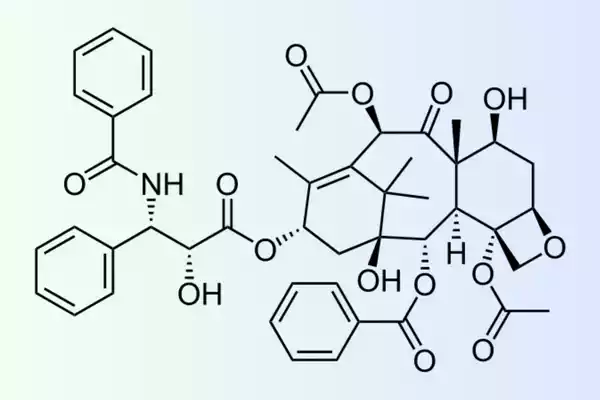
breast, ovarian Kaposi’s sarcoma, and other AIDS-related Kaposi’s. The pharmacological effect of paclitaxel can be attributed to its diterpenoid complex molecule (C47H51NO14) which has the taxane ring system.
It is with a four-ring structure that is fused. Paclitaxel contains a range of functional groups within its chemical structure, such as isomers, hydroxyl groups an amide group, and an oxetane ring. Functional groups are attached to microtubules and alter their dynamic and block cell division from affecting their biological function.
The hydrophobic nature of paclitaxel presents issues in its preparation and use. It is initially combined together with Cremophor EL (poly-oxyethylene casting oil) and ethanol to increase the solubility. But, other formulations, like the albumin-bound form of paclitaxel (nab-paclitaxel) and nab-paclitaxel, have been developed to increase solubility and minimize negative consequences.
What is Docetaxel?
Docetaxel is a different chemotherapeutic drug that belongs to the same taxane class of drugs. The drug is a semi-synthetic drug made from precursors found within the needles from the European yew tree (Taxus baccata).
Docetaxel is also a blocker of the function of microtubules in cells, which can hinder cell division and limit the growth of cancerous cells. It is often given intravenously to treat prostate, breast, and non-small cell lung cancer.
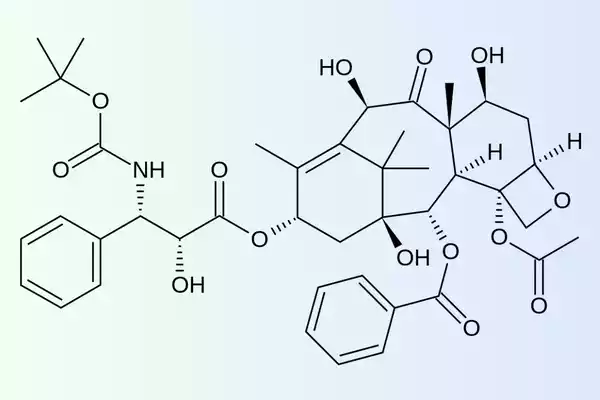
Docetaxel’s chemical structure is extremely complex. Its molecular formula chemically is C43H53NO14. Docetaxel is also an elongated taxane ring that has many functional groups like ester, hydroxyl, as well as amide, which is like the compound paclitaxel.
Docetaxel, in addition, is hydrophobic, making formulation and administration challenging. So, polysorbate 80 and alcohol are usually added to the formulation to improve solubility.
In recent times, new delivery strategies and formulations including nanoparticle formulations, liposomal formulations, albumin-bound docetaxel, and prodrugs have been designed to increase their solubility and bioavailability, as well as drug delivery efficiency, and undesirable side effects.
What is the Difference Between Paclitaxel and Docetaxel?
Both docetaxel and paclitaxel hinder cell division and also exert their anticancer effects by interfering with the microtubule’s function. There is however an important distinction in Paclitaxel as well as Docetaxel.
Paclitaxel is a product of the Pacific yew plant and is synthesized docetaxel originates from the chemical precursors from the European Yew tree. There are various formulations, including Paclitaxel typically using a Cremophor EL and an ethanol vehicle, and docetaxel utilizing polysorbate 80.
Paclitaxel is utilized for a variety of cancers as adjuvant or first-line treatment, whereas docetaxel is predominantly used for metastatic or advanced breast lung, and prostate cancers. In terms of their mechanism in action,
it is known that paclitaxel impacts the mitotic spindle within the G2 and M phases while docetaxel affects the centrosome structure and affects cells in the S/G2/M phase. They show different pharmacokinetics, affinity for b-tubulin receptors, uptake and rate of efflux, reactions that are hypersensitive, and toxicity patterns.
Table:
| Aspect | Paclitaxel | Docetaxel |
|---|---|---|
| Chemical Structure | Taxane ring with unique side chains | Modified structure with additional oxygenation and tert-butyl ester group |
| Solubility | Poor water solubility requires Cremophor EL and ethanol | Improved water solubility, formulated with polysorbate 80 and ethanol |
| Mechanism of Action | Stabilizes microtubules, binds to β-tubulin interface | Stabilizes microtubules, binds to a distinct site on β-tubulin |
| Clinical Indications | Ovarian, breast, and non-small cell lung cancers | Metastatic breast, prostate, and gastric cancers |
| Dosage and Sequencing | Different dosing and administration protocols | Different dosing schedules and adjustments |
| Side Effect Profile | Unique hypersensitivity reactions | A different set of side effects, potentially better tolerated |
| Resistance Patterns | Can develop resistance over time | Some cross-resistance with paclitaxel, but may overcome resistance in certain cases |
Chemical Structure and Formulation?
Paclitaxel and docetaxel belong to the taxane class of drugs, with distinct chemical structures within this classification. Paclitaxel possesses a complex diterpenoid base characterized by an extended taxane ring while docetaxel has oxygenation as well as a tert-butyl ester group for additional oxygenation and solubilization, their structural differences impact formulation,
solubility, and administration procedures within clinical environments, Cremophor EL or ethanol is often needed for solubilization while polysorbate 80 and ethanol can make docetaxel more water-soluble than its hydrophobic counterpart,
These differences also play a part in solubilization processes which in turn affect administration procedures as well as side effect profiles, Understanding differences between these taxanes is paramount when applied within clinical settings.
Clinical Applications?
Both Docetaxel and Paclitaxel have their own individual clinical uses, yet overlap in treating various cancers. Paclitaxel is most frequently employed for breast, ovarian, and non-small cell lung cancer treatment as monotherapy or in combination,
Docetaxel stands out as being used against metastatic prostate, breast, and stomach cancers in addition to non-small-cell lung cancer. Although their indications overlap significantly, their distinct dosages and effects impact treatment sequences and adjustments,
understanding these specifics allows physicians to tailor treatments specifically tailored toward specific patients for maximum efficacy while minimizing adverse side effects.
Current Research
- Nanotechnology Applications: Crafting nanocarrier systems that will enhance drug delivery while decreasing adverse side effects and defeating drug resistance is one area of research being pursued today.
- Combination Therapy: Investigating synergistic effects of taxanes when combined with immunotherapies, targeted therapies, or any other chemotherapeutic agent.
- Pharmacogenomics: Examining genetic variations that impact individual responses to taxane treatment with the goal of developing tailored dosing strategies.
- Discovering Resistance Mechanisms: In-depth exploration of molecular pathways related to taxane resistance is being performed, in an attempt to find effective strategies that may allow us to overcome or reverse such resistance.
- Tumor Microenvironment Taxanes: Understanding their interactions with both tumor microenvironments and immune responses could result in more efficient treatments.
- Alternative Formulations: Conceiving new formulations that reduce adverse side effects associated with traditional formulations while increasing tolerance and adherence can be done through alternative designs.
Future Future Directions
- Targeted Delivery Systems: Constructing taxane delivery methods that precisely target cancer cells while sparing healthy tissues from damage and decreasing any possible negative side effects or risks of adverse reactions is one future direction of cancer therapy.
- Precision Medicine: Employing information specific to each patient – such as tumor characteristics and genetic profiles – in order to customize taxane treatment and achieve better results.
- Enhance Therapeutic Index: To increase or maintain the efficiency of taxanes by mitigating their toxic side effects and thus permitting higher doses with improved results for patients.
- New Indications: Exploring whether taxanes could help combat other forms of cancer or non-cancer conditions by exploring their mechanism of action.
- Taxanes as Adjunctive Therapies: Researching taxanes as complement treatments alongside newer options like cell therapy and gene therapies.
- Biomarker Development: Finding reliable biomarkers that accurately reflect patient responses to taxane therapy for use in selecting and monitoring treatment regimens.
Ethical and Regulatory Considerations for Market Participants
- Patient Safety: Prior to using any new therapies or formulations clinically, ensure all have been rigorously assessed for safety testing.
- Informed Consent: Discussing potential risks and benefits associated with experimental taxane therapies and obtaining informed consent from participants who participate in clinical trials.
- Regulation Approval: Utilizing regulatory pathways to bring innovative taxane formulations, delivery systems, or treatment techniques to market.
- Fair Access: Ensuring access to advances in taxane treatments regardless of socioeconomic standing or location is of utmost importance.
The Final Outline: Paclitaxel vs. Docetaxel
Taxanes have been recognized as potent chemotherapy agents that have demonstrated a broad range of their activities. Clinical trials that have been conducted in a randomized manner have demonstrated the efficacy of paclitaxel as well as docetaxel as members of the taxane group,
For treating a variety of adjuvant and metastatic cancers which has highlighted their value as potent anti-tumor drugs. Paclitaxel comes from the Pacific yew tree and is synthesized, and docetaxel is made from precursor compounds found in the European yew tree.