Azo and Diazo: Diazo and azo differ primarily in that the latter refers to any substance with an N=N group while diazo refers specifically to organic substances containing an azo group in their center. Diaazo and Azo are terms commonly encountered in organic chemistry. “Azo” refers to compounds with the functional group N=N while those situated closer to the end of molecules are known as diazo compounds.
Definition of Azo compounds
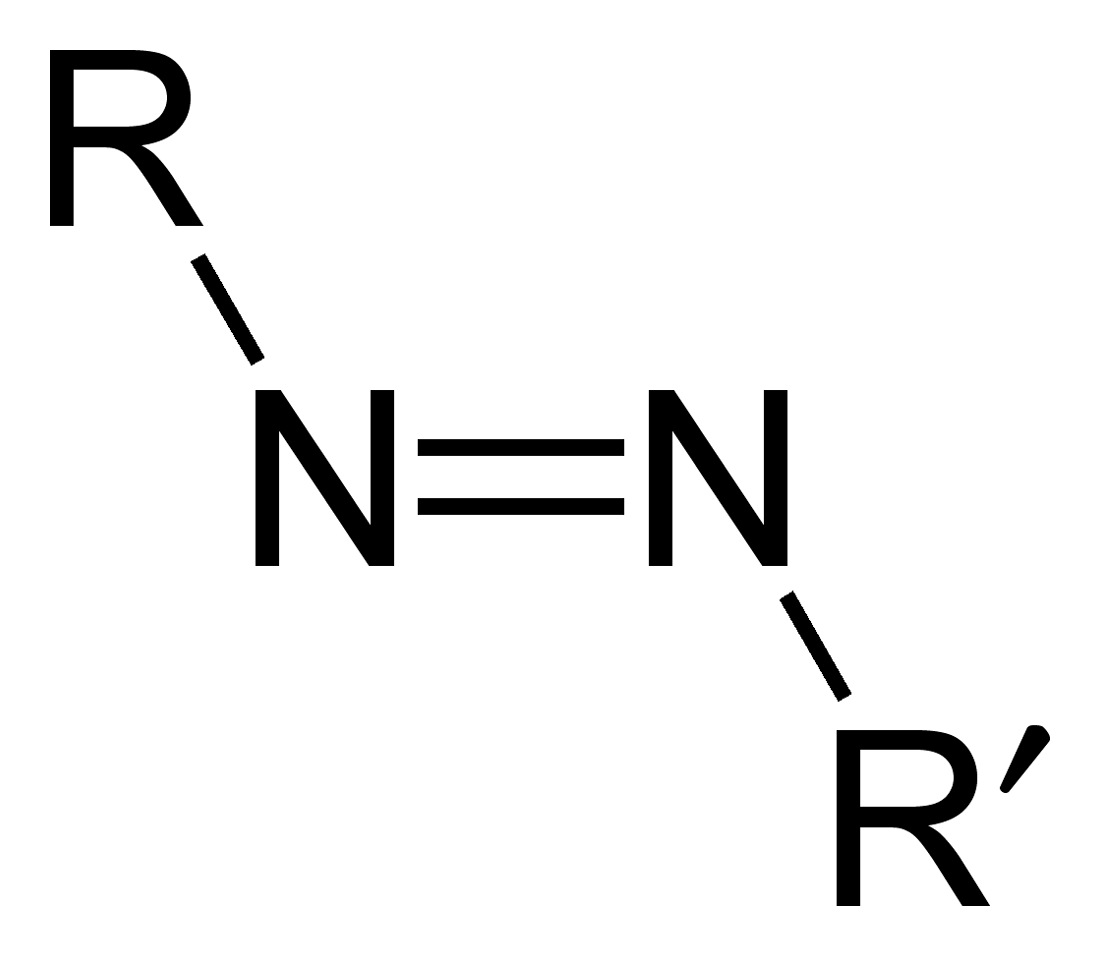
Azo compounds belong to an organic class of compounds with the characteristic azo group, comprised of two nitrogen atoms joined via two bonds. Azo compounds can be distinguished from other organic molecules by their bright and colorful hues due to conjugated double bonds in their molecular structures; as a result, they are widely used in many industries such as pigment production and dye manufacturing processes as well as pharmaceutical formulation. Synthesis methods used include diazotization reactions or coupling reactions depending on the chemical structure used and substituents present.
Definition of Diazo compounds
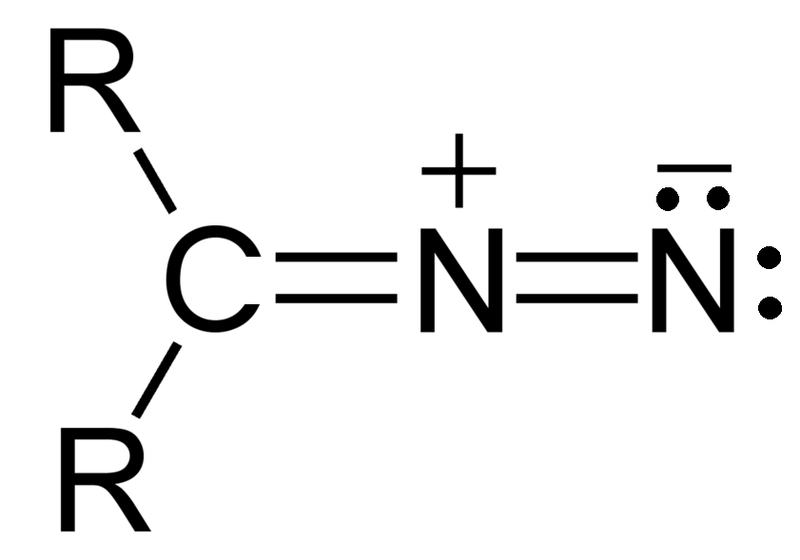
Diazo compounds belong to a class of organic compounds known as diazo compounds that feature the diazo group (-N=N+). This composition features two nitrogen atoms bound via double bonds and one attached directly to a substitute atom. Diazo compounds are notoriously reactive due to the diazo group, making them ideal precursors in organic syntheses. Their diazo group facilitates various transformations such as nucleophilic substitutions, cycloadditions, and rearrangements.
Diazo compounds can be produced through either reactions between primary amines and nitrosating agents or diazotization reactions between primary aromatic amines and nitrogenous acid, or diazotization reactions between aromatic primary amines and diazotization reactions using nitrogenous acid. They have numerous applications in organic synthesis for carbon-carbon bond formation as well as in photolithography and pharmaceuticals fields.
Importance of understanding the difference between Azo and Diazo compounds
Understanding the differences between Azo Diazo and Diazo compounds is vitally important for several reasons, including:
- Chemical Structure: Azo and Diazo compounds each possess their own distinctive chemical structures, with Azo compounds featuring nitrogen-nitrogen double bonds (-N=N-), while Diazo compounds feature diazo groups (-N=N+). Understanding their differences will assist with accurately classifying and identifying compounds.
- Stability and Reactivity: Azo and Diazo compounds possess distinct chemical reactions and properties; with Azo compounds being generally more stable than Diazo compounds due to having less reactive diazo groups present in them. Understanding these distinctions is vital when trying to select suitable reactions, and understanding their behavior as substances involved in chemical reactions.
- Methods of Synthesis: Azo and Diazo compounds can be produced through various techniques. Azo compounds typically involve coupling reactions while diazotization reactions typically create them. Understanding these techniques enables an individual to devise effective and tailored methods of producing these compounds.
- Application: Azo and Diazo compounds have multiple applications across industries. Azo compounds are widely utilized as pigments, dyes, and polymer additives due to their vivid colors and long-lasting qualities; while diazo compounds find use as flexible intermediates used to form numerous functional groups and carbon-carbon bonds. Understanding their respective uses helps identify which compound would best meet a particular need.
- Safety Considerations: Diazo compounds pose greater safety risks compared with Azo compounds due to their higher level of reactivity, so understanding their characteristics of reactivity is vital in order to safely store and use them while taking the appropriate precautions to minimize risk.
At the core of it, all lies essential knowledge for chemists, researchers, and professionals in industries that deal with dyes pigments pharmaceuticals and organic syntheses. Knowing which compounds belong to which class enables informed decisions on reactions selection chemical conditions application suitability for safer more efficient procedures within this field of chemistry.
Azo Compounds Details
Azo compounds belong to the class of organic compounds which contain an azo group as their main functional group, such as N=N+ or O=O+.
Here are a few key features associated with them:
- Structure of Azo Compounds: Azo compounds consist of two nitrogen atoms joined through two bonds (-N=NN). Azo groups can be found in numerous chemical situations and may connect to various molecular backbones.
- Synthesis: Synthesizing Azo compounds involves several techniques such as diazotization reactions and coupling reactions. Diazotization involves reacting an aromaticamine with nitrogen acids to produce diazonium salt which then undergoes coupling reactions by using appropriate coupling agents and produces the Azo compound.
- Properties: Azo compounds are well known for their vibrant and vivid shades, spanning orange to red to yellow to blue to green and beyond. Their distinguishable hue is due to conjugated double bonds in their structure which play an essential role in their color. Furthermore, azo compounds possess unique solubility properties due to molecular structures and functional groups found within their molecular structures.
- Reactivity: Reactivity Azo compounds undergo various reactions, such as reduction, oxidation, and substitution reactions. Their reactions could be altered by adding electron-donating and electron-drawing substituents in their azo group or on its molecular backbone.
- Application: Azo compounds find multiple uses in the dye and pigment industry, from plastics and textiles to inks and paints. Azo dyes are well known for their vivid and durable hue, making them an excellent addition to plastics, textiles, and paints alike. Furthermore, azo compounds are employed in manufacturing anti-inflammatory and antibiotic drugs; additionally, they can also be added as polymer additives to improve properties like fire retardancy and UV resistance.
Organic chemicals have an invaluable role to play as coloring agents and functional materials in numerous industries such as textiles, pharmaceuticals, and polymers.
Diazo Compounds Details
Diazo compounds are an organic class of molecules containing diazo groups (-N=N–).
Some key features related to Diazo compounds:
- Structure: Diazo compounds consist of two nitrogen bonds connected by double nitrogen bonds (-N=N+), each connected to an atom of nitrogen linked with an organic substituent such as alkyl, aryl, or any other organic group.
- Synthesis: Diazo compounds can be synthesized using various techniques. A popular one is diazotization, in which an aromatic amine is combined with nitrous acids to form diazonium sodium salts which may further undergo reactions such as decomposition or coupling in order to produce diazo compounds.
- Reactivity: Diazo compounds are highly reactive due to the presence of diazo groups. They undergo numerous reactions, from nucleophilic substitution and cycloaddition reactions to rearrangements and decomposition processes; making diazo-based compounds essential intermediates in organic syntheses.
- Application: Diazo compounds play an integral part in organic synthesis. As versatile elements for creating complex organic molecules, diazo compounds can be utilized in multiple reactions to introduce functional groups or create carbon and carbon bonds that are new. They have particular significance when applied to producing pharmaceuticals, agricultural chemicals, or specialty chemicals.
- Photolithography: Diazo compounds play an integral part in photolithography, an industry-standard technique utilized in microelectronics and printing industries. Commonly referred to as photoresists, diazo compounds react when exposed to UV radiation by photodecomposing and creating patterns on surfaces – an easy way to transfer pictures or designs onto them.
Diazo compounds must be handled carefully due to their high level of reactivity, but their flexibility makes them extremely valuable tools in organic synthesis and industrial applications.
Key Differences between Azo and Diazo Compounds
There are several key differences between Azo Diazo and Azo Diazo compounds:
- Structural Difference: Azo compounds contain double bonds of nitrogen and nitrogen (-N=N–), while diazo compounds have diazo groups made up of both double-bonded nitrogen-nitrogen molecules and another nitrogen atom attached to an adjacent substitute nitrogen atom.
- Reactivity: Azo compounds tend to be more stable and resistant to chemical reactivity compared with Diazo compounds, due to the latter’s diazo groups being highly reactive and capable of catalyzing numerous chemical transformations such as nucleophilic substitution, cycloaddition, and rearrangements due to being highly labile groups.
- Methods of Synthesis: Azo compounds are typically synthesized via coupling reactions between aromatic amines to form one azo compound. Diazo compounds, however, typically arise through diazotization reactions which involve the transformation of aromatic amines into diazonium sulfate that can then be modified into diazo compounds.
- Applications: Diazo compounds, with their high degree of reactivity, find use primarily in organic synthesis as key intermediates in creating functional groups as well as carbon-carbon bonds in organic molecules. They may also serve as polymer additives. Azo compounds are popularly employed in the pigment and dye industries due to their vibrant hues; they’re also often seen used as polymer additives or in pharmaceutical manufacturing processes.
- Stability: Stability Azo substances tend to be more stable than Diazo compounds, with Azo compounds typically being more resistant to decomposition or chemical reactivity than Diazo compounds. Diazo compounds, on the other hand, tend to be more reactive and unstable requiring special handling procedures and reactive conditions in order to produce acceptable reactions.
Chemical scientists, researchers, and other professionals working with Azo and Diazo compounds must have an in-depth knowledge of their key distinguishing features to effectively identify, classify and predict these substances’ reactivity as well as locate suitable applications across industries. This helps in the identification and classification process, selecting suitable synthesis techniques, and finding applications in diverse fields.
Differences between Table:
| Aspect | Azo Compounds | Diazo Compounds |
|---|---|---|
| Functional Group | Azo group (-N=N-) | Diazo group (-N=N-) |
| Structural Difference | Contains a nitrogen-nitrogen double bond and no additional atom | Contains a nitrogen-nitrogen double bond and an extra nitrogen atom |
| Reactivity | Generally less reactive | Highly reactive |
| Stability | More stable under normal conditions | Less stable, requiring careful handling |
| Synthesis Methods | Coupling reactions | Diazotization reactions |
| Color Properties | Known for vibrant and diverse colors | Can be colorless or used as intermediates for colored compounds |
| Applications | Dyes, pigments, pharmaceuticals, polymer additives | Organic synthesis, the creation of complex molecules |
| Usage in Industries | Textiles, paints, inks, plastics | Pharmaceuticals, specialty chemicals, microelectronics |
| Safety Considerations | Relatively lower safety risks | Require precautions due to reactivity and potential hazards |
| Example Compound | Sudan Red, Methyl Orange | Diazomethane, Phenyl diazoacetate |
Similarities between Azo and Diazo Compounds
Though Azo and Diazo compounds differ significantly, there are certain similarities among them that should not be ignored.
- They include: Nitrogen-Nitrogen Double Bond Azo and Diazo compounds contain nitrogen-nitrogen double bonds (-N=N-). This feature of their structures contributes to their reactivity, having an impactful influence on their properties and reactions.
- Usage in Various Industries: Both Azo diazo and Azo compounds have wide-ranging applications across industries. Azo compounds are particularly popular with pigment and dye producers due to their vibrant hues; while Diazo compounds provide flexible intermediates in organic synthesis for producing more complex organic compounds.
- Function In Organic Reactions: Both Azo and Diazo compounds play an integral part in organic reactions as reactive intermediates, with Azo compounds typically participating in coupling reactions while Diazo compounds provide nucleophilic substitutes, rearrangements, cycloadditions and many other changes that take place within organic systems.
- Importance of Coloration: Both Azo and Diazo compounds possess characteristics of color. Azo compounds are especially popular due to their vibrant and varied shades, making them excellent pigments and dyes. Although diazo compounds don’t naturally possess hues themselves, they may serve as intermediates in creating colored compounds through additional reactions.
- Reactivity: Both Azo and Diazo compounds exhibit Reactivity due to an interaction between nitrogen and carbon double bonds, though Diazo compounds tend to be less reactive than Azo compounds which can undergo different reactions depending on their structural features and substituents.
Understanding these similarities will assist in building relationships between Azo and Diazo compounds while emphasizing their similarities in features and functions. But it is equally essential to grasp their individual differences as these can have significant effects on the composition, stability, reactivity, and specific uses.
Conclusion
Azo, as well as Diazo compounds, are key components in the field of organic chemistry, both with their own unique properties and uses. Azo compounds are coveted for their vivid colors and are widely utilized for textile production. However, Diazo compounds’ high reaction rate makes them an excellent intermediate for a myriad of chemical reactions. Understanding the distinct characteristics of these compounds is vital to harnessing their potential across different industries.

